Microsurgical lung transplantation in rats has allowed us to obtain new knowledge about lung transplantation. However, some aspects in human transplantation technique still have not been included in this model, which could interfere with the clinical interpretation and extrapolation of results.
MethodsTwenty left lung transplantations were performed with a cuff technique and technical modifications, such as brain death induction, the control of ischemia time and retrograde perfusion in the donor and the controlled sequential reperfusion of the implanted lung in the recipient.
ResultsSurvival rate was 80%. The transplanted lungs showed proper perfusion and ventilation with good permeability of the anastomoses. Signs of ischemia-reperfusion injury were observed in all animals while mild acute rejection was seen in half of them.
ConclusionsThe shown model proves valid and is very similar to the procedure carried out in humans, which would reduce the number of possible variables derived from the surgical technique when extrapolating the study results to clinical use.
El trasplante microquirúrgico de pulmón en ratas ha permitido adquirir nuevos conocimientos sobre el trasplante de pulmón. Sin embargo, algunos aspectos de la técnica de trasplante en humanos aún no han sido incluidos en este modelo, lo que podría interferir en la interpretación clínica y en la extrapolación de los resultados.
MétodoSe han realizado 20 trasplantes pulmonares izquierdos con la técnica de manguitos (cuff) incorporando algunas modificaciones técnicas como la inducción de la muerte cerebral, el control del tiempo de isquemia, la perfusión retrógrada en el donante y la reperfusión secuencial controlada del pulmón implantado en el receptor.
ResultadosLa supervivencia ha sido del 80%. Los pulmones trasplantados mostraron una adecuada perfusión y ventilación con buena permeabilidad de las anastomosis. Se han observado signos de isquemia-reperfusión en todos los animales, y de rechazo agudo leve en la mitad de ellos.
ConclusionesEl modelo que presentamos es válido y similar al procedimiento que se realiza en humanos, lo que reduciría el número de posibles variables derivadas de la técnica quirúrgica a la hora de extrapolar los resultados a la clínica.
Lung transplantation (LT) is a therapeutic alternative for some terminal respiratory diseases. It is currently indicated in advanced pulmonary emphysema, cystic fibrosis, diffuse interstitial disease and pulmonary hypertension. The most widely used technique is bilateral lung transplantation, followed by single lung transplantation. The importance of improving certain aspects, such as preservation, ischemia time, ischemia reperfusion (IR) injury, acute rejection (AR) and chronic rejection (CR) makes it necessary to develop adequate experimental LT models.1
In the beginning, the most commonly used experimental animal model was dog,2,3 although the ethical and economic problems made researchers look for alternative models, such as rabbits4 and rats. In 1971, Asimacopoulos5 started experimental LT in rats, and in 1982 Wildevuur6 completed the technique successfully, although with difficulties for performing the vascular and bronchial anastomoses with sutures.
The complexity of the microsurgical technique in rats improved in 1989 with the initial technique described by Mizuta.7 It was the first time that Teflon cuffs were used for performing the anastomoses. To create these, polyethylene intravenous catheters were used, cut into 2-mm cylinders. Initially, Mizuta performed the vascular anastomoses in the receptor with cuffs, while the bronchial anastomosis was done with sutures. Later, Mizuta8 and Reis9 reported doing all the anastomoses with cuffs. The modification in the technique significantly reduced the duration of the procedure and post-op mortality, which gave rise to the introduction of a new model for studying LT and its complications.
Since then, some authors10,11 have described modifications in the surgical technique for studying different aspects of LT and improving experimental conditions, but without including important aspects related with clinical LT, such as brain death (BD), retrograde perfusion in the donor or the start of ischemia time in these models.
In our study, we present a new anesthesia protocol and some modifications in the preservation and lung implantation technique in rats with the purpose of reproducing what is currently done humans. Our objective is to reduce possible variations in the surgical technique for the interpretation and extrapolation of the results.
MethodsAnimalsForty Sprague-Dawley male consanguineous rats (20 donors and 20 receptors) with a weight between 300 and 400g were housed under optimal conditions. All the animals were treated in accordance with the “Guidelines for the use and care of laboratory animals” published by the Spanish National Health Institute (Instituto Nacional de la Salud). The study was approved by the Ethics Committee for Animal Experimentation and Well-being at our hospital.
Induction of Brain DeathThe animals (n=20) were anesthetized with 0.25mg/kg subcutaneous (sc) medetomidine (Domtor®, Pfizer, Madrid, Spain) and 50mg/kg intramuscular (im) ketamine (Ketolar 500®, Pfizer, Madrid, Spain). The anterior part of the donor animal was shaved; orotracheal intubation was done with an Abbocath 14G catheter and the animal was ventilated with an Evita 4 ventilator (Dräger-Hispania, Madrid, Spain) with tidal volume (VT) 10ml/kg or plateau pressure (PPLAT) 12–14cmH2O and inspiration/expiration (I/E) ratio 1:2, respiratory rate (RR) 60rpm, fraction of inspired oxygen (FiO2) 0.21 and positive end-expiratory pressure (PEEP) 2cmH2O.
For the induction of BD, a 2-mm cranial trepanation was performed in the area anterolateral to the bregma and a Fogarty 3F catheter was introduced (LeMaitre® vascular, Burlington, MA, USA). BD was gradually induced with increased intracranial pressure by injecting a quantity of 20μl12 of saline solution per minute until 200μl were reached within the catheter balloon, which caused herniation of the cerebral trunk (Fig. 1). Body temperature was maintained close to 36.5°C using an electric thermal cushion. BD was confirmed by the presence of apnea, disappearance of electroencephalographic activity and dilated pupils.12,13
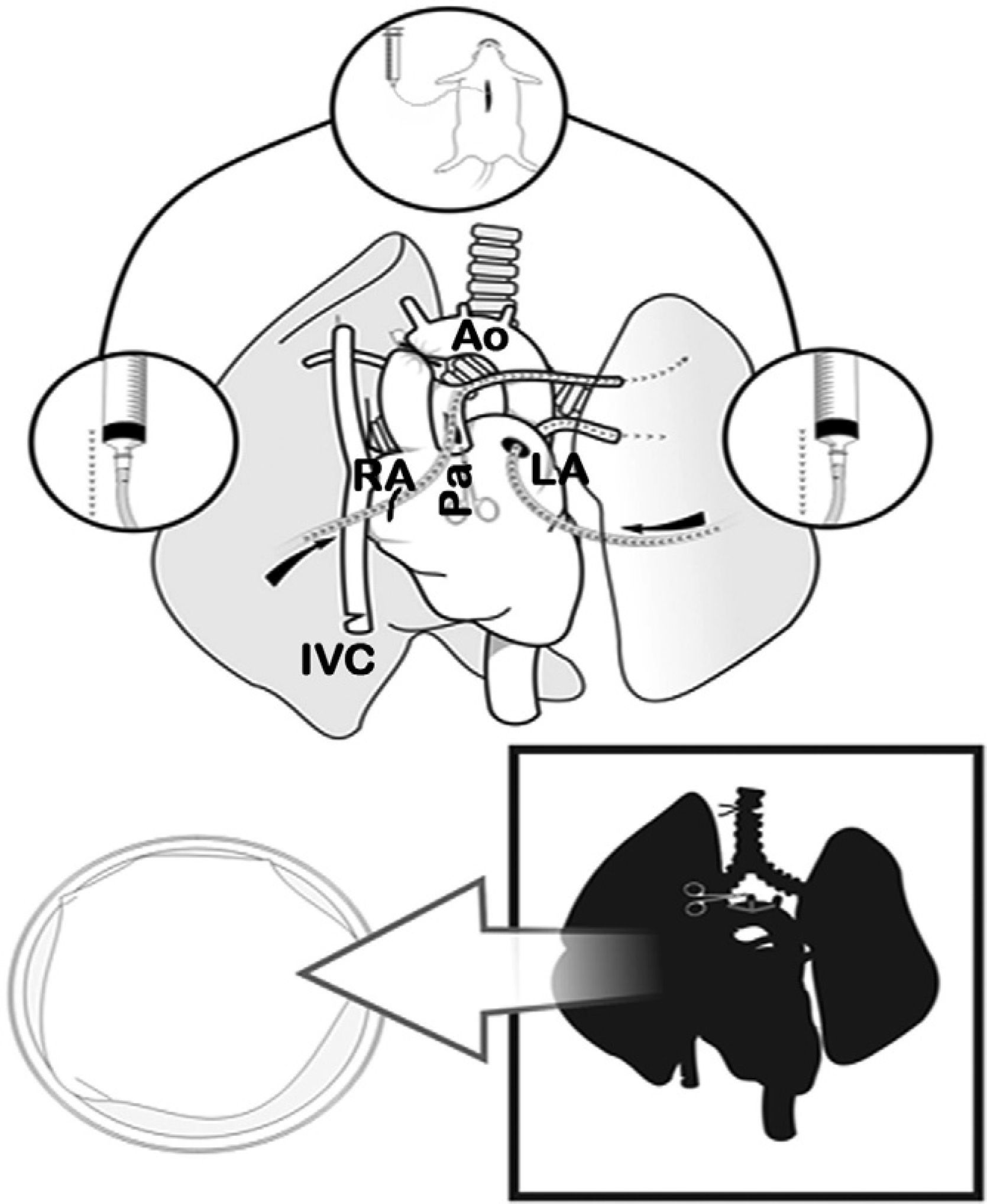
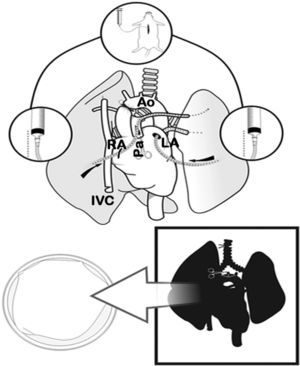
Lung Extraction and PreservationWith the rat in supine position, 100IU/100g of intravenous sodium heparin (Heparina sódica®, Chiesi, Barcelona, Spain) were injected into the jugular vein. Later, both subxiphoid window and mid-sternotomy were performed, opening the thorax wide with two Kocher clamps. Afterwards, we proceeded with the exeresis of the thymus and the dissection of the ascending thoracic aorta. The left auricle was cut and the ascending aorta was clamped with a permanent 4mm microclamp (Yasargil®, Medicom, Germany), initiating ischemia time (period between the interruption of the blood flow with the clamping of the aorta and the blood reperfusion of the lung through the artery after anastomosis). Next, the right auricle and the lower thoracic vein were cut and the thoracic cavity was filled with ice. When this caused cardiac arrest, the lungs were perfused with an anterograde cold preservation solution of low-potassium dextran glucose (LPDG) (Perfadex®, Kungsbacka, Sweden), with 500μg/l of prostaglandin E1 (Alprostadil®, Pfizer, Madrid, Spain) diluted in the bag through an incision in the base of the cone of the pulmonary artery using an Abbocath® 16G catheter (16–20ml of LPDG at a height of 30cm). Afterwards, retrograde perfusion was carried out through the left auricle (10–12ml of LPDG from 20cm high).
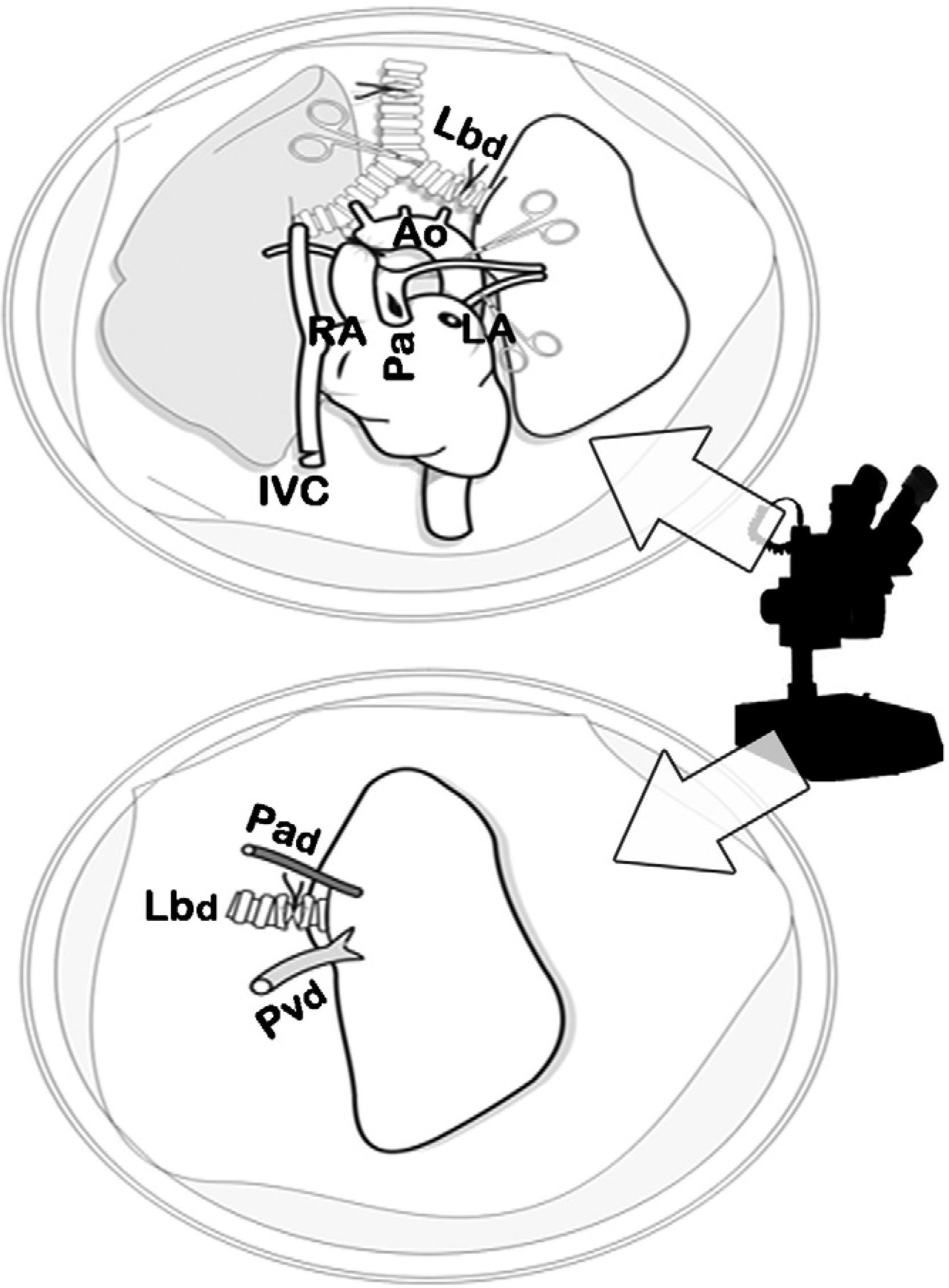
When the perfusion of the lungs was homogenous, we proceeded with the ligation and transection of the trachea with the lung semi-inflated at 50% of total lung capacity (10–15cmH2O), the extraction of the cardiopulmonary block with traction separating it from the esophagus and the transection of the supra-aortic trunks, thoracic aorta, vena cava and the pulmonary ligaments. The cardiopulmonary block was placed on a Petri dish with cold LPDG, and we dissected the artery, left bronchus and vein using a 10× optical microscope (Carl Zeiss®, Germany) with transection distal to the hilum of said structures for separating the left lung. A microclamp was placed in the bronchus to maintain the lung in a state of semi-inflation (Figs. 2 and 3).
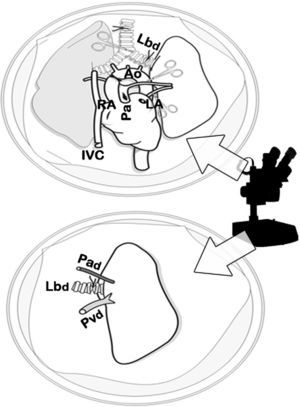
Clamping of the thoracic aorta (Ao) and transection of the left auricle (LA), right auricle (RA) and the inferior vena cava (IVC). Transection of the pulmonary artery (Pa) and anterograde perfusion. Afterwards, retrograde perfusion through the left pulmonary vein and auricle, and finally tracheal ligature with lungs semi-inflated and cardiopulmonary extraction.
Dissection of the cardiopulmonary block by optical microscope. Dissection of the artery (Pad), vein (Pvd) and left bronchus (Lbd) of the donor and transection of the structures distal to the hilum for the separation of the left lung, prior to the closure of the bronchus with a microclamp in order to maintain the lung in a state of semi-inflation.
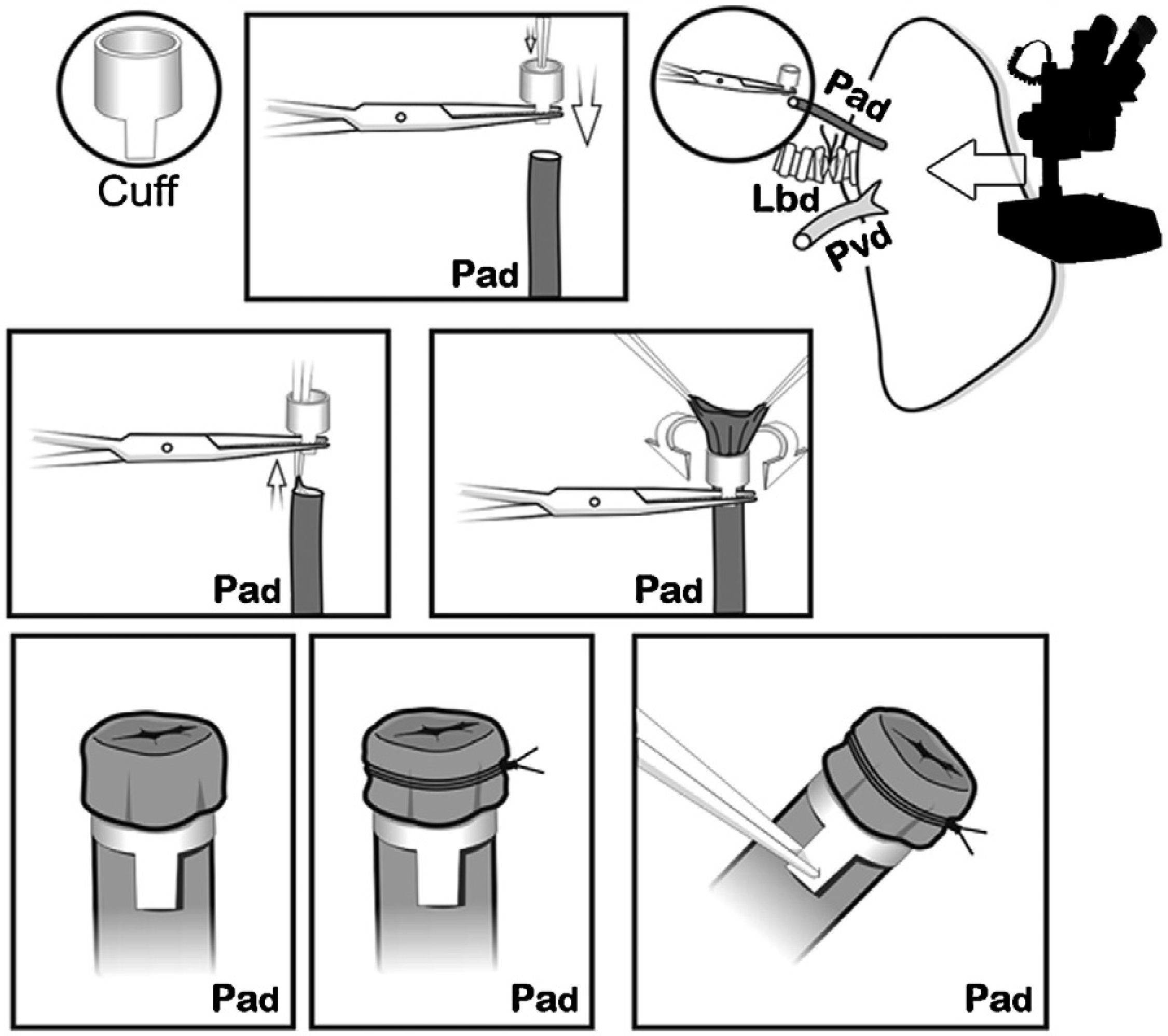
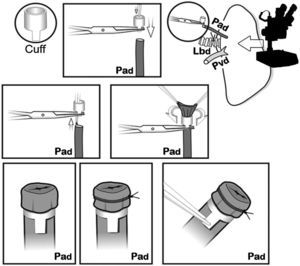
The cuffs were constructed with an Abbocath® 16G catheter (1.7mm in diameter). Each cuff had a body and a 1.5mm-long tab. The tab was used to hold the cuff with a clamp during the implantation. First, the pulmonary artery was passed through the cuff. The proximal end was everted over the cuff and held in place firmly with a non-absorbable 7/0 ligature (Fig. 4). The same process was carried out with the bronchus and the pulmonary vein, respectively, and then the lung was stored in cold LPDG until the moment of its implantation, in our case 2h after clamping the thoracic aorta.
Implantation in the ReceptorThe receptor animals (n=20) were anesthetized with 0.25mg/kg medetomidine sc, 50mg/kg ketamine im and 0.7mg/kg atropine im (Atropine®, Braun, Barcelona, Spain). Once anesthetized, the rats were intubated with an Abbocath® 14G. The receptor animals were ventilated in the same manner as the donors.
After shaving the left hemithorax, left thoracotomy was carried out in the fourth intercostal space and the lung was removed from the thoracic cavity by means of a metallic paper clip. The pulmonary hilum was dissected in order to identify the artery, the bronchus and the pulmonary vein. The bronchial arteriolar plexus was cauterized together with the arteriole of the pulmonary ligament, under the vein. Long 6/0 polypropylene ligatures were placed, as well as microclamps (Yasargil® 4mm) on each one of the structures, as close as possible to the heart. The artery and vein were cut proximally and tangentially to the heart and an intravascular lavage was done with heparinized plasma. Later, the bronchus was also transected.
The donor lung was placed on the native lung of the recipient and was frequently irrigated with a cold preservation solution until the anastomoses were completed with the cuffs. In doing so, each cuff was introduced into the corresponding structure through the orifice made. Once inside, it was set in place transitorily with a microclamp placed over the tab in order to facilitate the definitive anastomosis with ligatures, and then the microclamp was removed.
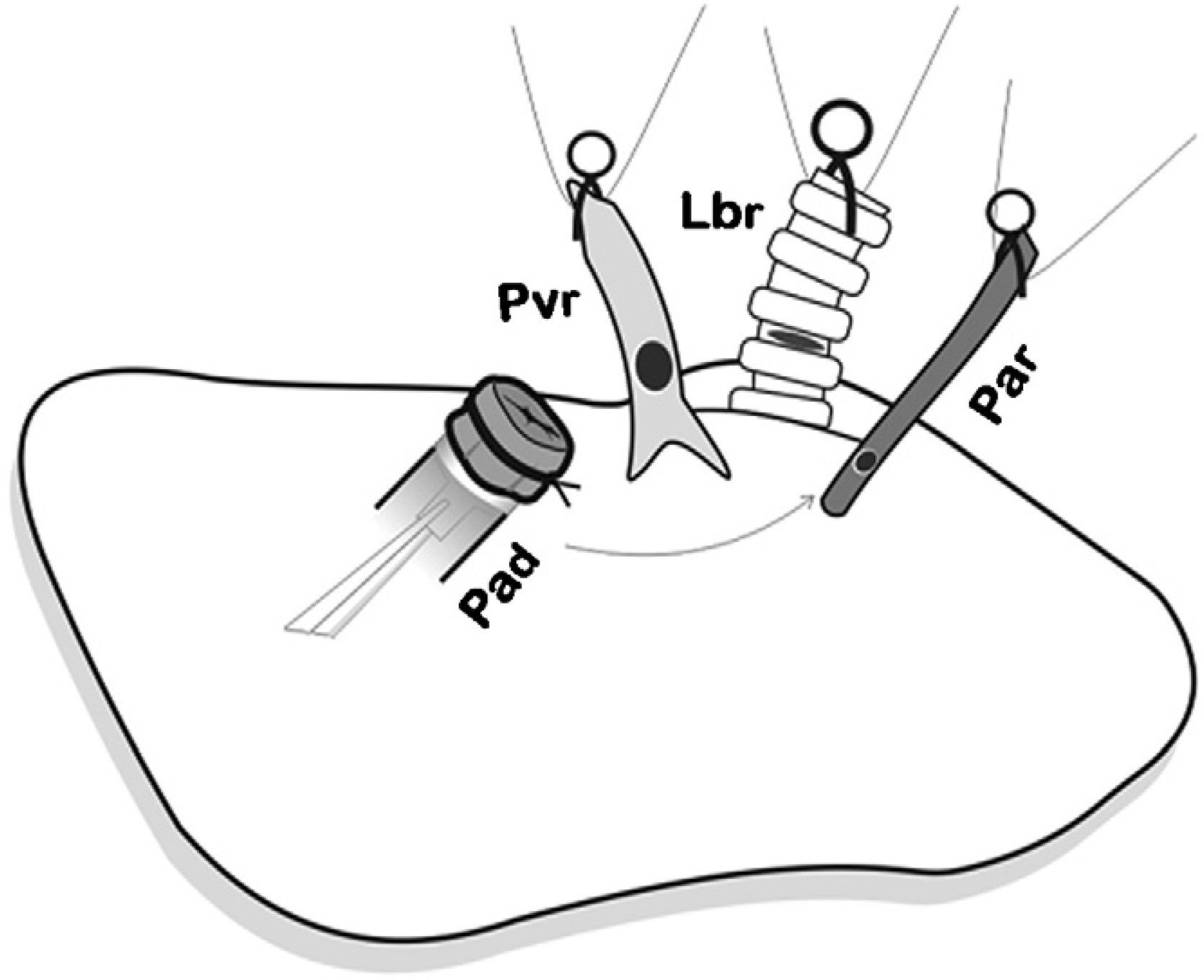
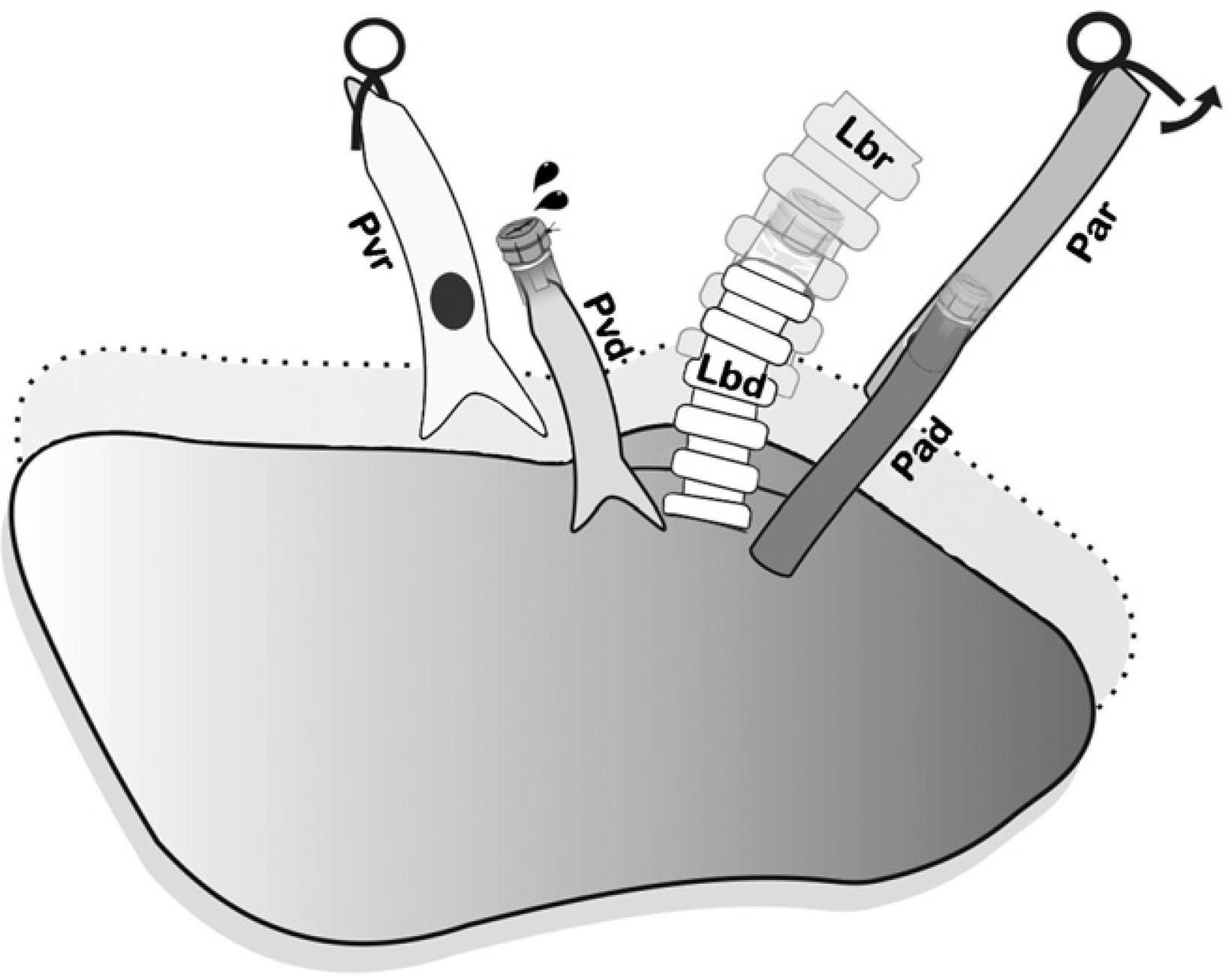
First of all, the arterial anastomosis was done, followed by the bronchial (Fig. 5), and the sequential reperfusion of the donor lung was completed. The microclamp proximal to the heart was withdrawn transitorily from the bronchus to allow for the ventilation of the lung with a PEEP of 5cmH2O eliminating atelectasis. Then, the pulmonary artery was slowly reperfused by opening the microclamp proximal to the artery until it was observed that a few drops of blood flowed out the pulmonary vein (Fig. 6). The artery and the bronchus were clamped once again and we proceeded with the anastomosis of the vein. Slow, progressive reperfusion was carried out by opening the vein microclamp, then the bronchus and finally the artery.
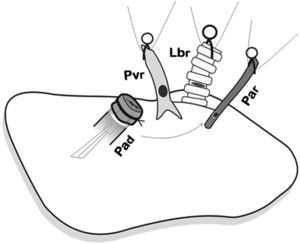
Placement of the donor lung on the recipient lung and introduction of the cuffs on each of the corresponding structures and later anastomosis. First, the arterial anastomosis was done. Pad: pulmonary artery of the donor; Par: pulmonary artery of the receptor; Lbr: left bronchus of the recipient; Pvr: pulmonary vein of the receptor.
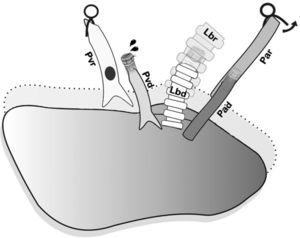
Controlled anterior sequential reperfusion. The clamp was removed from the bronchus and the lung was ventilated transitorily with PEEP at 5cmH2O in order to eliminate atelectasis, later slowly reperfusing from the pulmonary artery until some drops of blood come out the pulmonary vein of the donor. Pad: pulmonary artery of the donor; Par: pulmonary artery of the receptor; Lbd: left bronchus of the donor; Lbr: left bronchus of the recipient; Pvd: pulmonary vein of the donor; Pvr: pulmonary vein of the receptor.
Once the donor lung was implanted, the native lung was removed from the receptor and an 8-Fr pleural drainage catheter was inserted and connected to syringe for aspirating, and the thoracotomy was closed. Afterwards, we administered 0.25mg/kg im atipamezole (Antisedan®, Pfizer, Madrid, Spain), an antagonist of medetomidine, and 0.05mg/kg sc buprenorphine (Buprex®, Schering-Plough, Madrid, Spain). When the rat began spontaneous breathing, the thoracic and orotracheal tubes were withdrawn.
Post-op Follow-up and Evaluation of the TransplantationRats that survived surgery were sacrificed under general anesthesia with 0.25mg/kg sc medetomidine and 50mg/kg im ketamine at 7 (n=8) and 90 days (n=8). After clamping the artery and the right pulmonary bronchus, the cardiopulmonary block was removed with the lung inflated. The block was set in formaldehyde through the trachea and was included in paraffin for the histological study. In our case, the animals received no immunosuppressant treatment during the study period.
The samples obtained from the transplanted lung were stained with hematoxylin–eosin. Signs of IR injury were evaluated, such as alveolar interstitial edema, vascular congestion, alveolar hemorrhage and neutrophilic infiltration, and the IR injury was classified as mild, moderate or severe. AR signs were also evaluated, such as the distribution of the perivascular and peribronchial mononuclear infiltrate in accordance with the International Rejection Classification, as were signs of CR, such as bronchial and bronchiolar dilation, areas of vasculitis and fibrosis. The anastomoses were also inspected visually in order to determine their macroscopic permeability.
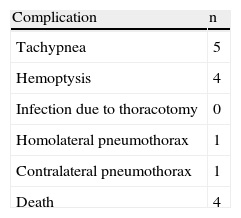
From a clinical standpoint, we evaluated the presence of tachypnea, hemoptysis, respiratory failure and post-surgical infections during the post-op period. In all cases, chest radiographs were taken at 55kV and 3.2mA (Siemens Mobilett II®, Siemens España, Madrid, Spain) immediately after surgery as well as 7 and 90 days, before the animal was sacrificed.
ResultsMean surgical time was 48.5±2.0min for the extraction and 59.2±4.2 for the implantation. Mean time for the complete procedure was 107±6.2min.
No post-op infections were observed. Four of the recipient animals (two from the 7-day group and two from the 90-day group) presented tachypnea and hemoptysis due to severe edema and died within the first 48h. One animal presented tachypnea for the first 24h. The other animals presented no clinical problems, and the survival rate was 80% (Table 1).
In the histologic study, signs of IR injury were observed after 7 days in all the transplanted lungs (n=8): mild (n=5), moderate (n=2) and severe (n=1); signs of AR were seen in 6 animals (A1=3 and A2=3). After 90 days, in all the animals (n=8) a fibrotic lung stump was observed, and microscopically we viewed bronchial dilation with areas of vasculitis and fibrosis accompanied by severe AR (A4), indicating the presence of active CR. No histological changes were observed in the non-transplanted lung. The inspection of the cuffs showed in all animals adequate bronchial and vascular lumen without signs of stenosis or thrombosis.
Chest radiography showed adequate lung expansion with an increase in density of the transplanted lung related to the presence of IR injury after 7 days and CR after 90. One animal presented mild left pneumothorax and another presented contralateral pneumothorax immediately after surgery, with no clinical repercussions.
DiscussionLT is still a challenge for patients and medical transplantation groups, and there are still important areas requiring basic and clinical research. To face these challenges, the American National Heart, Lung, and Blood Institute14 (NHLBI) recommends considering the following points: (a) to identify essential biomarkers, based on proteomic and genomic studies that can predict the presence of CR; (b) to develop relevant animal models that better reproduce human diseases in order to research the pathogenesis of lung injury after transplantation; and (c) to support and promote research covering the range of graft dysfunctions, including CR.
In this direction, following the recommendations of the NHLBI, the experimental model that we have developed in this study is based on previous reports of cuff techniques7–9 with modifications in the preservation and implantation techniques such as the induction of BD, control during the ischemia time and retrograde perfusion in the donor and controlled sequential reperfusion in the lung of the recipient, in addition to an improvement in the percentage of survival of 80% compared with other transplantation models in rats.11 This is an attempt at developing animal models that reflect what happens in human diseases according to the aforementioned international recommendations.
The organs used for transplantation come mainly from BD donors.15 The majority of the donors suffer considerable damage in the central nervous system due to subarachnoid hemorrhage or traumatic brain injury before being declared BD.16 BD induces autonomic and hemodynamic alterations as well as alterations in the neurohormonal state of the donor17,18 that can induce lung edema and intra-alveolar hemorrhage and should be taken into account in experimental LT models in order to simulate a model that is closer to reality.
Different animal models have been described that evaluate the effects caused in donated organs due to either rapid-onset or gradual-onset BD13,16,18,19 used in transplant studies for kidneys,20 liver21 and very rarely in LT22 (Table 2). In our case, we have used the slow BD model, with a gradual increase of the intracranial pressure in order to reduce the hemodynamic instability of the donor13 and the subsequent degree of lung edema, which in the case of rats is aggravated by the lability of the pulmonary vascular permeability. In fact, in donor BD rats, a severe pulmonary edema is frequently produced more than 2h after the induction of BD; this is why we do the LT with 2h of ischemia time.22
Evolution of the Lung Transplantations in Rats.
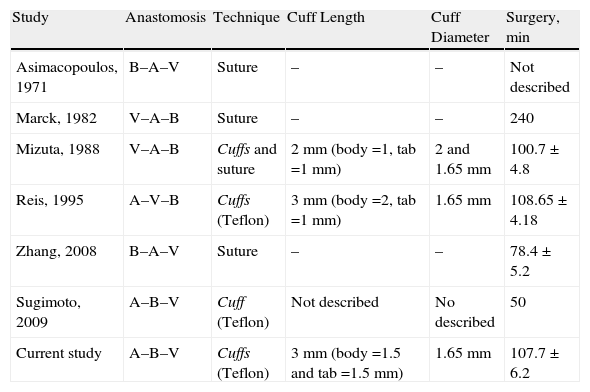
| Study | Anastomosis | Technique | Cuff Length | Cuff Diameter | Surgery, min |
| Asimacopoulos, 1971 | B–A–V | Suture | – | – | Not described |
| Marck, 1982 | V–A–B | Suture | – | – | 240 |
| Mizuta, 1988 | V–A–B | Cuffs and suture | 2mm (body=1, tab=1mm) | 2 and 1.65mm | 100.7±4.8 |
| Reis, 1995 | A–V–B | Cuffs (Teflon) | 3mm (body=2, tab=1mm) | 1.65mm | 108.65±4.18 |
| Zhang, 2008 | B–A–V | Suture | – | – | 78.4±5.2 |
| Sugimoto, 2009 | A–B–V | Cuff (Teflon) | Not described | No described | 50 |
| Current study | A–B–V | Cuffs (Teflon) | 3mm (body=1.5 and tab=1.5mm) | 1.65mm | 107.7±6.2 |
The anastomosis column shows the order of the anastomoses in the surgery (B: bronchus; A: pulmonary artery; V: pulmonary vein). The surgery time is expressed in minutes (average±standard deviation).
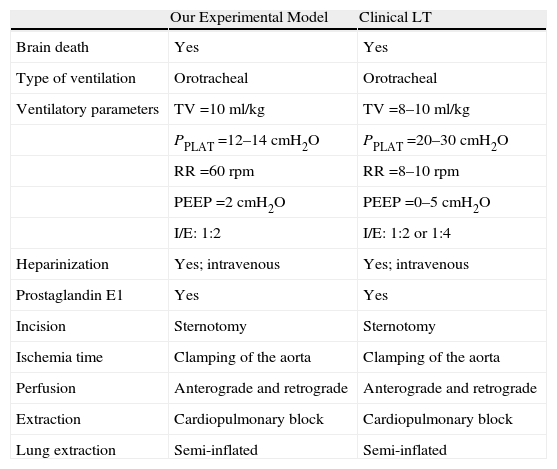
In our model, orotracheal intubation with an Abbocath® 14G minimizes peritracheal air leak and ensures better ventilation of the rat during surgery, as there are no endotracheal tubes for rats. The removal of the donor lung by mid-sternotomy,9,23 unlike Mizuta,7 facilitates the extraction of the thoracic organs and minimizes any possible injury. Similar to transplantation in humans, the clamping of the aorta gives us precise control of the duration of the ischemia, which is not controlled in other proposed models.7,9 This also acts as a mechanical factor favoring cardiac arrest, similar to what happens in humans. In our model, as in that by Reis,9 we prefer 16G cuffs in order to minimize the risk of tearing the hilar structures, especially the pulmonary vein. The reperfusion and permeability have been shown to be good; therefore, we consider them to be adequate for animals between 300 and 400g.
An important part of the extraction process in humans is the anterograde and retrograde organ preservation. During extraction, the flow volume and rate should guarantee homogenous perfusion of the lung. This should be carried out with the lungs ventilated in order to avoid areas of atelectasis and poor perfusion.24 In our model, we have reached optimal perfusion with an Abbocath® 16G using cold low-potassium dextran solution, which is currently the most widely used in clinical practice. The introduction of retrograde perfusion, which has not been reported in other rat models, is important because anterograde perfusion has been shown to be incomplete as it obviates the bronchial circulation.25 Retrograde preservation can be safely done with lower pressure and volume than those used in anterograde perfusion due to the pulmonary vascular lability of this type of animals. This makes our model more similar to what happens in clinical practice (Table 3).
Technical Comparisons Between the Lung Preservation in out Experimental Model and Lung Transplantation in Clinical Practice.
| Our Experimental Model | Clinical LT | |
| Brain death | Yes | Yes |
| Type of ventilation | Orotracheal | Orotracheal |
| Ventilatory parameters | TV=10ml/kg | TV=8–10ml/kg |
| PPLAT=12–14cmH2O | PPLAT=20–30cmH2O | |
| RR=60rpm | RR=8–10rpm | |
| PEEP=2cmH2O | PEEP=0–5cmH2O | |
| I/E: 1:2 | I/E: 1:2 or 1:4 | |
| Heparinization | Yes; intravenous | Yes; intravenous |
| Prostaglandin E1 | Yes | Yes |
| Incision | Sternotomy | Sternotomy |
| Ischemia time | Clamping of the aorta | Clamping of the aorta |
| Perfusion | Anterograde and retrograde | Anterograde and retrograde |
| Extraction | Cardiopulmonary block | Cardiopulmonary block |
| Lung extraction | Semi-inflated | Semi-inflated |
In our case, the extraction of the cardiopulmonary block is similar to what is done in humans, unlike what is described in other experimental models where only the left lung is extracted. This enables us to maintain the ventilation of the lung with FiO2 0.21 and PEEP 2cmH2O24,26 until the ligature of the trachea and extraction of both lungs semi-inflated to 50% of their total capacity, which is achieved with an air pressure of 12–15cmH2O. The dissection of the left hilum with a microscope minimizes the risk of vascular injury and enables the vessels and the bronchus to be cut as long as possible for the placement of cuffs and the later transplantation. The clamping of the left main bronchus before its transection at the carina allows us to maintain the lung ventilated during its preservation prior to implantation.27
The anesthesia protocol, especially in the receptor, allows for the animal to be awakened at any moment without it being necessary to metabolize the anesthesia, add extra doses or use inhaled anesthesia. The effects of medetomidine can be easily reverted with its antagonist, atipamezole.28 In our case, we associate it with ketamine in order to achieve proper sedation, relaxation and hypnosis. In addition, the administration of atropine diminishes the secretions of the bronchial tree and has palliative effects on the secondary effects caused by medetomidine.29 Due to the effect of the anesthesia, hypothermia may appear, which is controlled with an electric blanket under the animal; if it is not corrected, it may cause death. The administration of these three drugs has constituted in our experience a rapid, efficient and safe anesthesia protocol for LT in these animals.
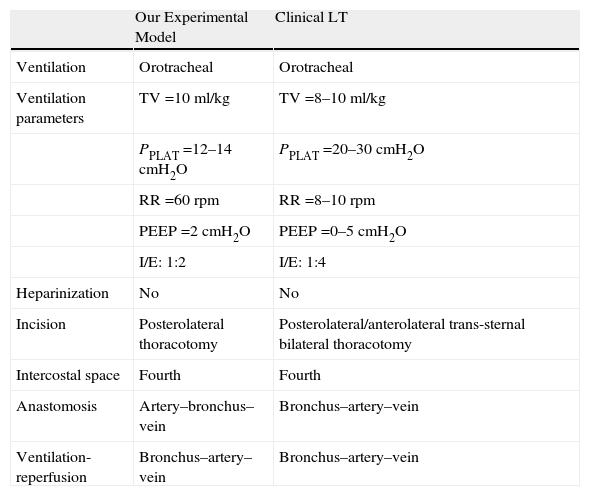
The approach of the thoracic cavity through the fourth intercostal space in the recipient (unlike other authors who use the fifth intercostal space9) allows us to better manipulate the pulmonary hilum for its dissection. In addition, the transection of the pulmonary vessels and the main bronchus as far as possible from the heart and on its anterior side,7 without completely cutting the structure, make the anastomoses much easier. Similarly, using long ligatures behind the microclamps makes it easier to introduce the cuff and close the anastomosis, unlike what is described by Reis.9
Controlled sequential reperfusion24,30 is a critical step in human transplantation, but it has not been described in other proposed experimental models. In our model, we carried out anterograde reperfusion with the ventilated lung, enabling a homogenous distribution of the blood flow of the lung with flushing of the preservation solution out the vein, similar to what happens during transplantations in clinical practice. In addition, it avoids the introduction of massive quantities of preservation solution within the vascular venous flow along with an increase in the lung capillary pressure and a subsequent pulmonary edema unrelated with IR injury, which may cause artifacts in the results (Table 4).
Technical Comparisons Between the Lung Implant in our Experimental Model and the Lung Transplant in Clinical Practice.
| Our Experimental Model | Clinical LT | |
| Ventilation | Orotracheal | Orotracheal |
| Ventilation parameters | TV=10 ml/kg | TV=8–10ml/kg |
| PPLAT=12–14cmH2O | PPLAT=20–30cmH2O | |
| RR=60rpm | RR=8–10rpm | |
| PEEP=2cmH2O | PEEP=0–5cmH2O | |
| I/E: 1:2 | I/E: 1:4 | |
| Heparinization | No | No |
| Incision | Posterolateral thoracotomy | Posterolateral/anterolateral trans-sternal bilateral thoracotomy |
| Intercostal space | Fourth | Fourth |
| Anastomosis | Artery–bronchus–vein | Bronchus–artery–vein |
| Ventilation-reperfusion | Bronchus–artery–vein | Bronchus–artery–vein |
Thus, we conclude that, with the modifications that we have described, the LT technique with cuffs in rats more closely resembles what happens in human clinical practice. This could facilitate the interpretation and clinical extrapolation of the results obtained by working with these experimental models, which are essential in LT research.
FundingThis project has been funded by the Spanish Health-Care Research Fund (Fondo de Investigación Sanitaria Español-FIS 07/01145) and by the Fundación MM in Madrid. Dr. Norberto Santana Rodríguez (INT 07/172) and Dr. Bernardino Clavo (INT 07/030) have worked under the Research Intensification Program (Programa de Intensificación de la Investigación-I3SNS) at the Carlos III Health Institute (Instituto de Salud Carlos III). This study has served as a foundation and continues thanks to a grant from the Carlos III Health Institute (FIS PI 10/01485).
We would like to thank Mr. Juan Verona and Mr. D. Ramón Saavedra for their technical help.
Please cite this article as: Santana Rodríguez N, et al. Modificaciones técnicas del modelo de trasplante pulmonar ortotópico en ratas con donantes en muerte cerebral. Arch Bronconeumol. 2011;47:488–94.