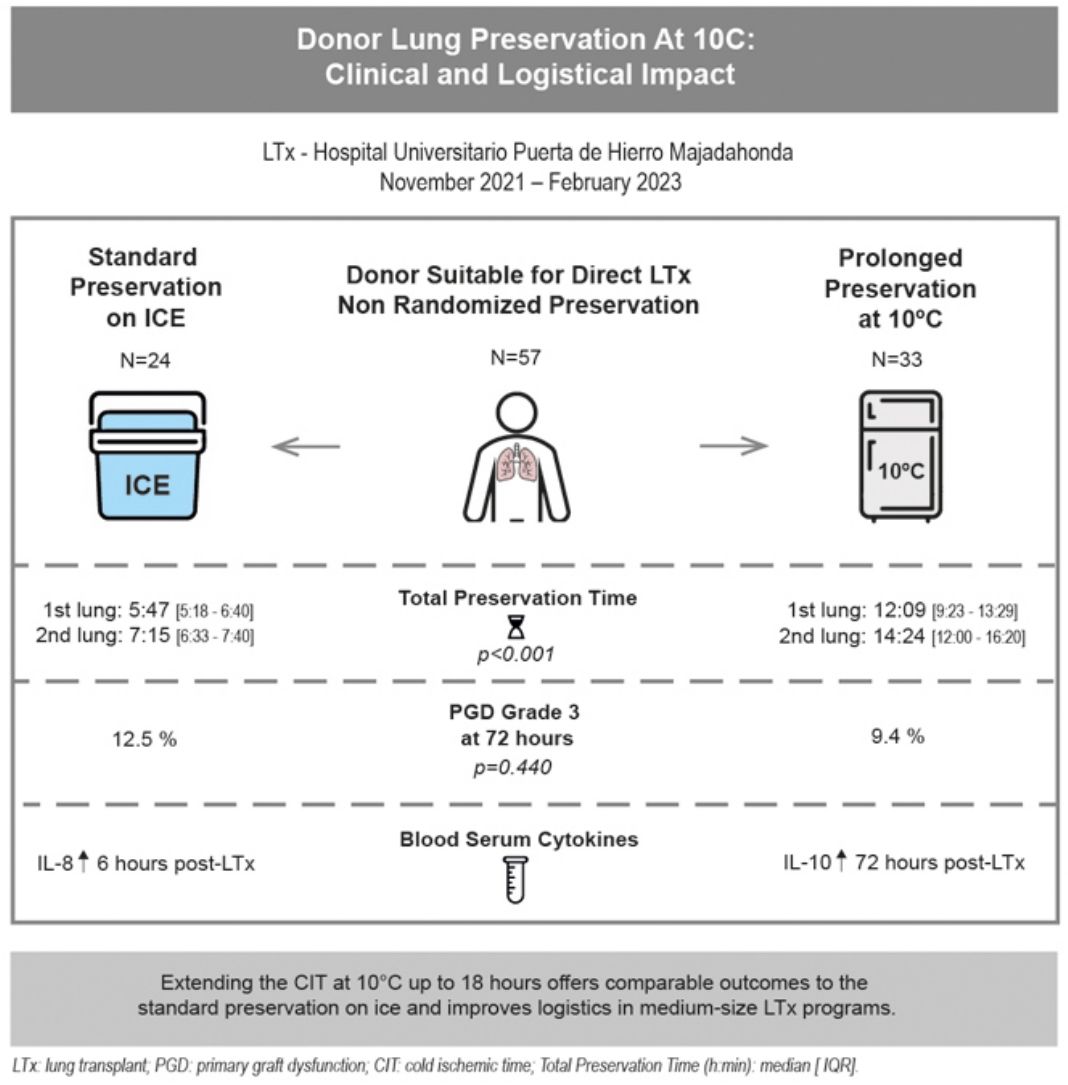
Cold static donor lung preservation at 10°C appears to be a promising method to safely extend the cold ischemic time (CIT) and improve lung transplant (LTx) logistics.
MethodsLTx from November 2021 to February 2023 were included in this single institution, prospective, non-randomized study comparing prolonged preservation at 10°C versus standard preservation on ice. The inclusion criteria for 10°C preservation were suitable grafts for LTx without any donor retrieval concerns. Primary endpoint: primary graft dysfunction (PGD) grade-3 at 72-h. Secondary endpoints: clinical outcomes, cytokine profile and logistical impact.
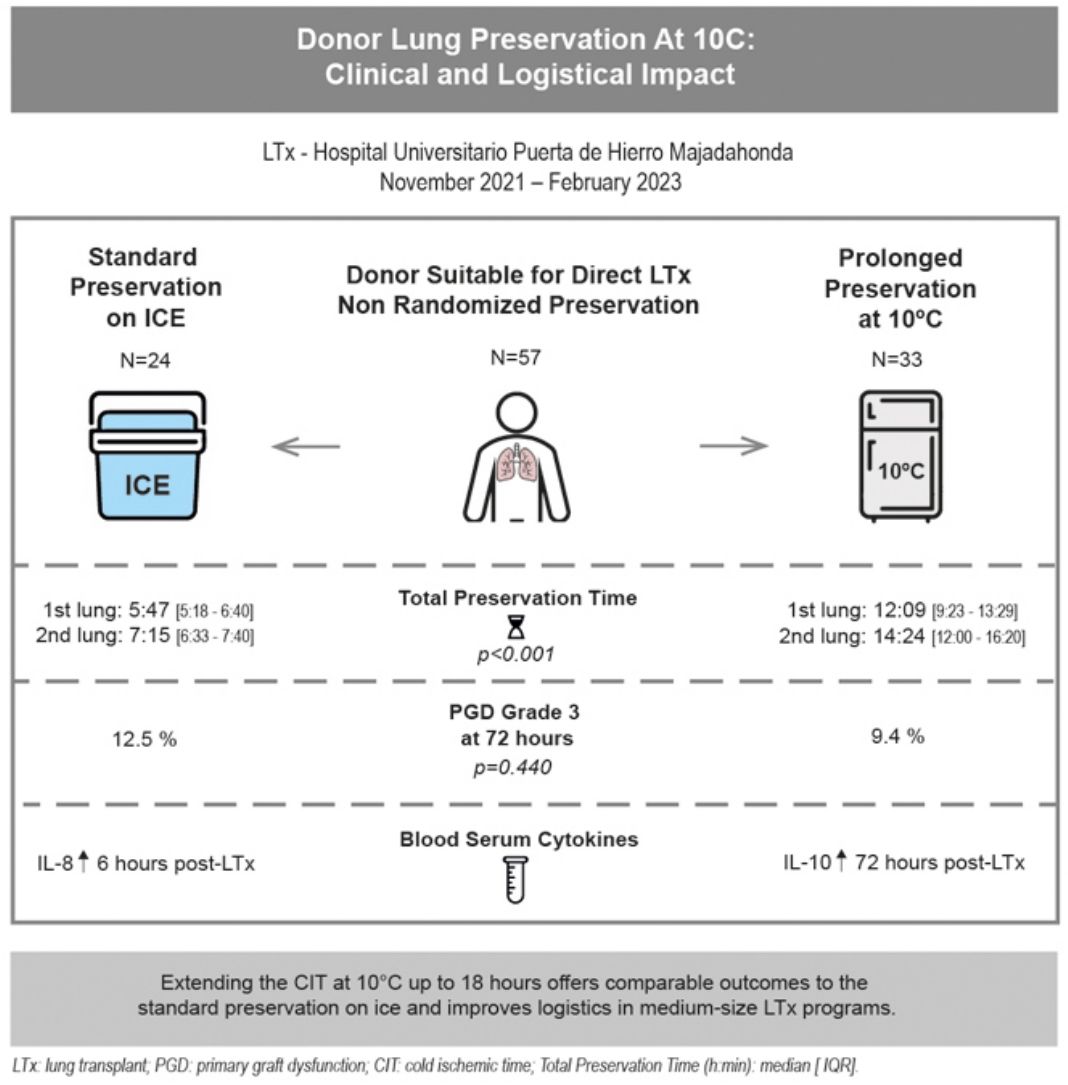
ResultsThirty-three out of fifty-seven cases were preserved at 10°C. Donor and recipient characteristics were similar across the groups. Total preservation times (h:min) were longer (p<0.001) in the 10°C group [1st lung: median 12:09 (IQR 9:23–13:29); 2nd: 14:24 (12:00–16:20)] vs. standard group [1st lung: median 5:47 (IQR 5:18–6:40); 2nd: 7:15 (6:33–7:40)]. PGD grade-3 at 72-h was 9.4% in 10°C group vs. 12.5% in standard group (p=0.440). Length of mechanical ventilation (MV), ICU and hospital stays were similar in both groups. Thirty and ninety-day mortality rates were 0% in 10°C group (vs. 4.2% in standard group). IL-8 concentration was significantly higher 6-h post-LTx in the standard group (p=0.025) and IL-10 concentration was increased 72-h post-LTx in the 10°C group (p=0.045).
ConclusionsPreservation at 10°C may represent a safe and feasible strategy to intentionally prolong the CIT. In our center, extending the CIT at 10°C may allow for semi-elective LTx and improve logistics with similar outcomes compared to the current standard preservation on ice.
The conventional approach for preserving lungs before transplantation involves flushing the organ with a low potassium dextran solution, keeping them inflated, and subsequently preserving them on ice.1,2 This preservation method is used for all solid organs due to its low cost and simplicity. However, the maximum preservation time for donor lungs using this method is typically limited to 6–8h.3
Preclinical studies conducted more than 30 years ago discovered that static lung preservation at 10°C was optimal.4,5 This concept of lung preservation at 10°C is being re-explored, given cytoprotective metabolites are produced and mitochondrial health is maintained when lungs are preserved at this temperature.6 Ali et al. investigated the effects of prolonged static cold storage on pig lungs exposed to 36h of static cold storage at either 10°C or on ice, followed by a 12-h evaluation on EVLP. The results showed that lungs stored at 10°C had less injury and improved pulmonary function compared to those stored on ice. Based on these findings, the concept of extending static cold preservation was tested clinically in five human patients with excellent outcomes.7 Preclinical data also published by the Toronto Lung Transplant Program suggested that the combination of 10°C storage with two cycles of 4-h normothermic EVLP may result in excellent graft function after a total of 3 days of preservation.8
A clinical trial has recently been conducted to evaluate the feasibility of using 10°C lung preservation to enable semi-elective LTx (ClinicalTrials.gov Identifier: NCT04616365).9 The primary endpoint of this study was to demonstrate the safety of 10°C lung preservation in a cohort of patients who underwent delayed transplantation, while avoiding nighttime procedures. The secondary endpoint was to compare the outcomes of these patients to those who underwent transplantation using standard preservation during a contemporaneous period of time. The study demonstrates that extended preservation at 10°C is both safe and efficient, resulting in low rates of PGD and showing comparable early and mid-term outcomes to those of lungs preserved using standard methods. The potential to extend cold static preservation time by up to 12–24h at 10°C could revolutionize the field of LTx.
The ability to reliably extend preservation periods could offer several benefits beyond avoiding overnight transplantation.10 These include overcoming current geographical limitations in organ donation, creating more time for immunological matching between donors and recipients, and the potential for performing LTx in a semi-elective manner. The successful implementation of semi-elective LTx has significant advantages, particularly in medium-size LTx programs, where both optimal OR scheduling and the well-being of health care professionals are important factors with regard to maintaining the surgical activity at the best possible level. Other advantages might include improved OR logistics or the acceptance of two simultaneous donors.11
Our study aims to compare the outcomes and the logistical impact of LTx using extended donor lung preservation at 10°C to the current standard of cold storage on ice.
Material and methodsStudy design and inclusion criteriaThis is a prospective non-randomized study including patients who underwent LTx between November 2021 and February 2023 at our center, comparing prolonged preservation at 10°C versus a simultaneous cohort of patients using standard preservation on ice.
Our study protocol was adapted from the recently published clinical trial evaluating the feasibility of using 10°C lung preservation to enable semi-elective LTx (ClinicalTrials.gov Identifier: NCT04616365).9
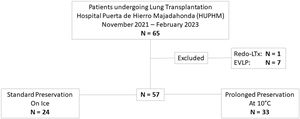
The donor inclusion criteria for prolonged preservation at 10C were lung grafts from both DBD or controlled DCD suitable for direct transplantation when expected crossclamp was later than 6:00pm. The parameters used for evaluating the donor lungs were P/F ratio, chest X-ray, bronchoscopy findings and donor history. The donor exclusion criteria were: any concern during graft procurement, doubts about the perfusion of the organ with the preservation solution, need for EVLP evaluation, an objective evidence of air leak that prevented the lungs from being inflated during the storage time, donor age greater than 70 years. Age was subsequently eliminated as exclusion criteria six months after the start of the study. The decision was based on an internal analysis of outcomes using donors older than 70 years old that did not show statistical significance when compared with younger donors.
The recipient inclusion criteria were patients older than 18 years old with signed informed consent and listed for primary lung transplantation. Redo lung transplantation or urgent status was considered as exclusions. However, after a few months, the investigators decided to eliminate the urgent status as an exclusion criteria because these patients might be more complex than elective cases (for instance, patients with extracorporeal membrane oxygenation (ECMO) as bridge to transplant12). Consequently, the study team decided to avoid overnight transplantation due this complexity.
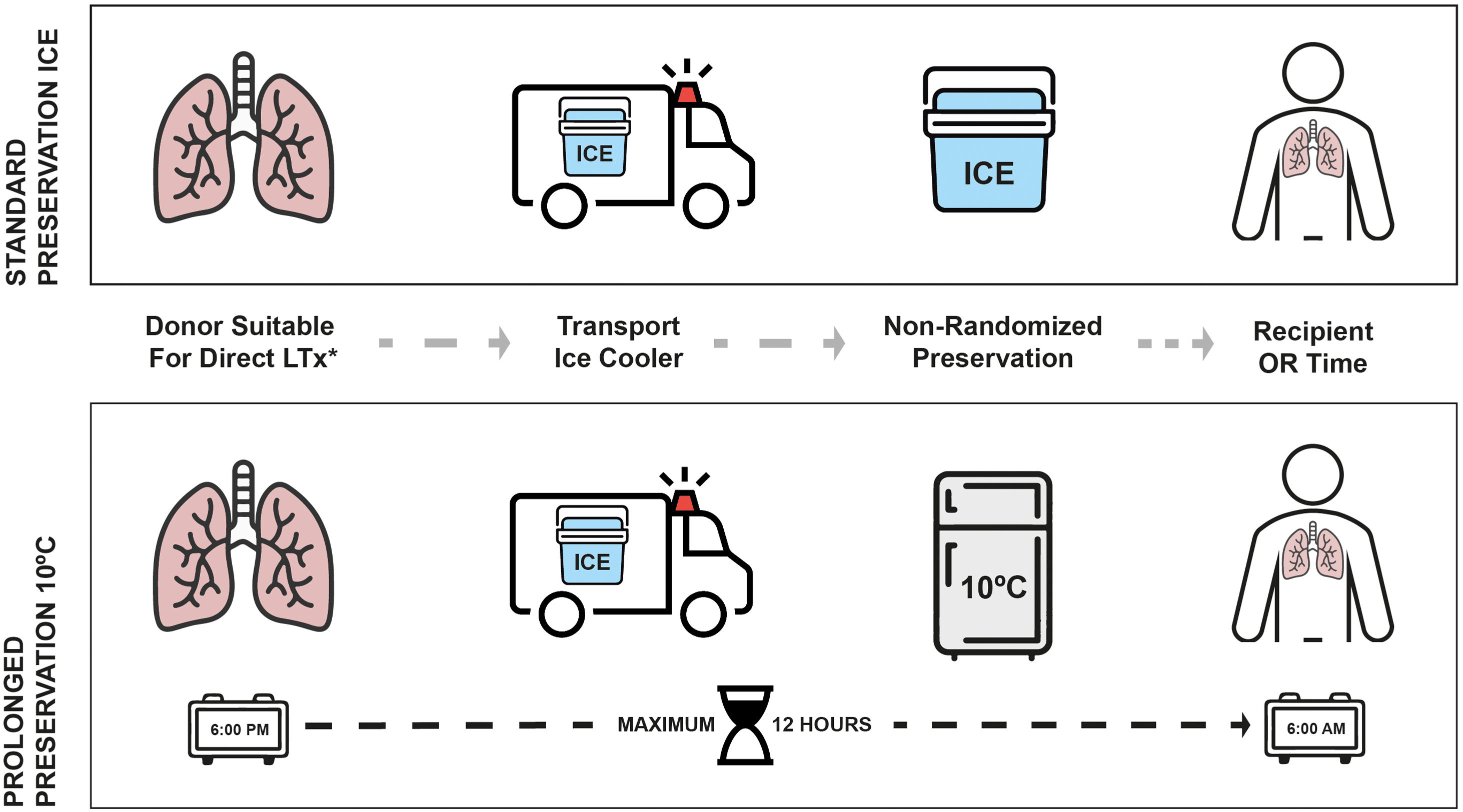
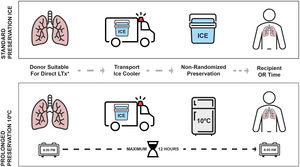
The decision of prolonged preservation at 10°C was made by the transplant team in order to intentionally extend the CIT for any logistical purpose once the retrieved grafts met the aforementioned criteria. Reasons for deciding to extend the CIT at 10C were the following: avoidance of night-time surgeries, delaying complex LTx cases to the morning, need for specific intervention that is only available during daytime or waiting for histopathological findings. On the other hand, reasons to follow the standard protocol on ice to go straight to transplant were: donor cross-clamp times before 6:00p.m., risk of lung deflation during prolonged storage, donor concerns, absence of informed consent, avoiding cancelation of morning elective cases and procurements performed by other programs.
Recipient selection was conducted according to blood type, size, and LAS, regardless of the elected preservation method.
Donor procurement and preservation proceduresAll lungs were retrieved from donors after brain death or after controlled circulatory death. Organ procurement was performed according to our standard protocols described elsewhere.13–15 Lung preservation was performed by anterograde pulmonary artery flushing with a low-potassium dextran-based solution after injecting prostaglandin E1 through the pulmonary artery. Subsequently, retrograde flush through the pulmonary veins was performed. Lungs were stored on ice after mild inflation with 50% oxygen and transported to the recipient hospital in the ice cooler.
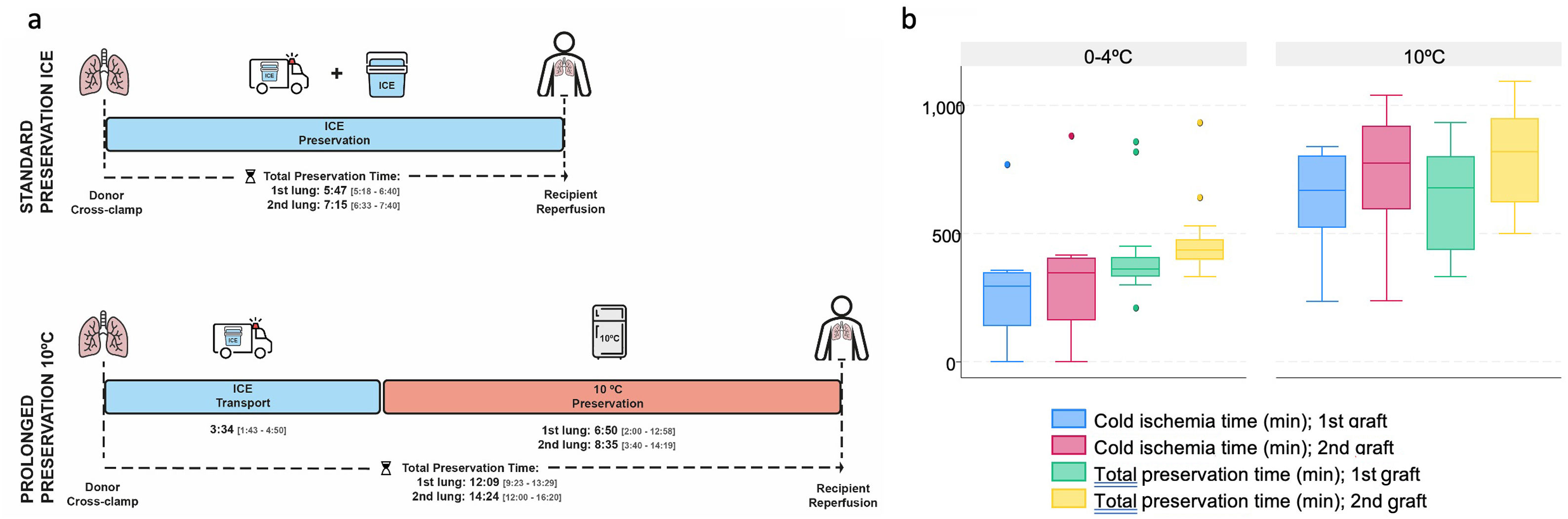
At the end of the retrieval, any grafts meeting the criteria for 10°C preservation and with a donor cross-clamp time after 6:00p.m. were transferred to a specific incubator located at the entrance of the recipient OR, which maintained uniform temperature at 10°C (MYTEMP™65HC, Benchmark Scientific, NJ, USA). There was a maximum of 12h from the donor cross-clamp time to the arrival of the recipient to the OR. Grafts following the standard preservation method were stored in the same ice cooler used for transport. Fig. 1 illustrates the study design and preservation procedures.
OutcomesThe primary endpoint was to compare the incidence of PGD grade 3 at 72h according to the 2016 ISHLT consensus,16 using different methods of preservation (10°C versus standard on ice). The secondary endpoints were the comparison of days of recipient MV, length of ICU and hospital stay, as well as 30 and 90-day mortality rates. We also performed a survival analysis of both groups.
The reasons for deciding upon prolonged preservation at 10°C or standard preservation on ice were also collected.
The histologic acute lung injury (ALI) score17 was analyzed comparing both preservation groups using lung biopsies performed 30-min after reperfusion. Our biopsy protocol includes a biopsy in the donor before cross-clamp, before implantation during graft preparation on the back table, and 30min to 1h after the reperfusion of that lung. The three samples are usually taken from the same lung and the same lobe (usually right middle lobe or lingula). We decided to focus on the 3rd sample because it was the most consistent biopsy. The ALI score evaluates three items (neutrophils, alveolar edema, and interstitial infiltrates), with each one receiving a score between 0 and 3 points, where 0 was no presence of the histological change, 1 was mild, 2 moderate and 3 severe (Table S1, Supplementary material).
The cytokine expression profile (Interleukin-8, Interleukin-10) was analyzed using ELISA (Enzyme-Linked Immunosorbent Assay) measuring the following blood serum samples: the recipient before transplant (T1); 6-h post-transplant (T4); 24-h post-transplant (T5); and 72-h post-transplant (T6).
Statistical analysisCategorical variables were analyzed using the Chi-square test while the Mann–Whitney U test was used for numerical variables. Overall survival was defined as the time from surgery to death from any cause. Patients who were lost to follow-up or who were alive at the end of the study were censored at the last visit date. Survival analysis was performed using Kaplan–Meier curves, and the comparison between the curves was performed via a log-rank test. The level of significance was set at 0.05. Stata v17 software was used for the analysis.
ResultsFrom November 2021 to February 2023, 57 LTx were performed at our institution (excluding redo-LTx and EVLP cases). Of these, 33 cases (57.9%) were transplanted after a prolonged preservation at 10°C, and 24 cases (42.1%) were preserved following the standard method on ice. Fig. 2 shows the study flowchart. The main reason for using 10°C storage was to intentionally extend the CIT to avoid overnight surgeries by performing LTx as a semi-elective procedure (N=20). Other logistical reasons for choosing prolonged preservation at 10°C included delaying complex LTx cases to the next morning (N=11), need for intraoperative plasmapheresis (N=1) or waiting for donor histopathological findings (N=1). Standard preservation on ice was carried out due to donor cross-clamp times before 6:00p.m. (N=9), donor concerns including a MSSA (Methicillin-sensitive Staphylococcus aureus) positive donor BAL and two cases with suspected air leak that might lead to deflation during the prolonged preservation storage (N=3), absence of informed consent (N=2), decision to avoid canceling morning elective OR schedule (N=6) or other reasons (3 urgent status cases prior to urgency being eliminated as an exclusion criteria and 1 case retrieved by another program [n=4]).
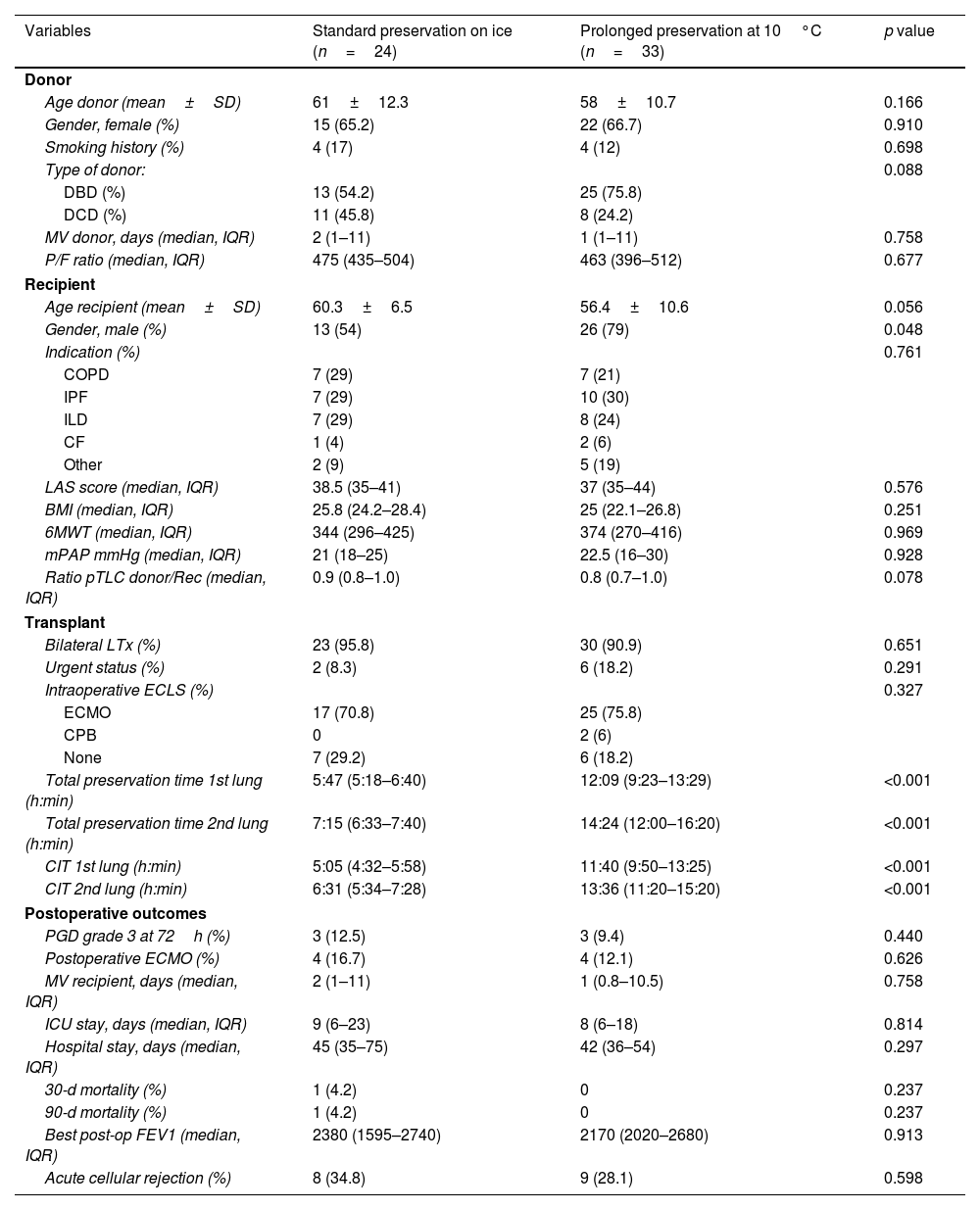
Donor and recipient characteristics were comparable between groups (Table 1). Most donors were female with a mean age of 58 years in the 10°C group vs. 61 years in the standard group. The donor P/F ratio was similar between groups (463 in the 10°C group vs. 475 in the standard group), as was the donor smoking history (12% in the 10°C group vs. 17% in the standard group). Seventy-nine percent of the recipients in the 10°C group were male (vs. 54% in the standard group; p=0.056). Bilateral transplants were performed in 90.9% cases in the 10°C group, and 18.2% in urgent status. Idiopathic Pulmonary Fibrosis (IPF) was the most frequent indication for LTx in both groups (30% in the 10°C group vs. 29% in the standard group), with a median LAS score of 37 and 38.5, respectively.
Demographics and transplant outcomes comparing prolonged preservation at 10°C vs. standard preservation on ice.
| Variables | Standard preservation on ice (n=24) | Prolonged preservation at 10°C (n=33) | p value |
|---|---|---|---|
| Donor | |||
| Age donor (mean±SD) | 61±12.3 | 58±10.7 | 0.166 |
| Gender, female (%) | 15 (65.2) | 22 (66.7) | 0.910 |
| Smoking history (%) | 4 (17) | 4 (12) | 0.698 |
| Type of donor: | 0.088 | ||
| DBD (%) | 13 (54.2) | 25 (75.8) | |
| DCD (%) | 11 (45.8) | 8 (24.2) | |
| MV donor, days (median, IQR) | 2 (1–11) | 1 (1–11) | 0.758 |
| P/F ratio (median, IQR) | 475 (435–504) | 463 (396–512) | 0.677 |
| Recipient | |||
| Age recipient (mean±SD) | 60.3±6.5 | 56.4±10.6 | 0.056 |
| Gender, male (%) | 13 (54) | 26 (79) | 0.048 |
| Indication (%) | 0.761 | ||
| COPD | 7 (29) | 7 (21) | |
| IPF | 7 (29) | 10 (30) | |
| ILD | 7 (29) | 8 (24) | |
| CF | 1 (4) | 2 (6) | |
| Other | 2 (9) | 5 (19) | |
| LAS score (median, IQR) | 38.5 (35–41) | 37 (35–44) | 0.576 |
| BMI (median, IQR) | 25.8 (24.2–28.4) | 25 (22.1–26.8) | 0.251 |
| 6MWT (median, IQR) | 344 (296–425) | 374 (270–416) | 0.969 |
| mPAP mmHg (median, IQR) | 21 (18–25) | 22.5 (16–30) | 0.928 |
| Ratio pTLC donor/Rec (median, IQR) | 0.9 (0.8–1.0) | 0.8 (0.7–1.0) | 0.078 |
| Transplant | |||
| Bilateral LTx (%) | 23 (95.8) | 30 (90.9) | 0.651 |
| Urgent status (%) | 2 (8.3) | 6 (18.2) | 0.291 |
| Intraoperative ECLS (%) | 0.327 | ||
| ECMO | 17 (70.8) | 25 (75.8) | |
| CPB | 0 | 2 (6) | |
| None | 7 (29.2) | 6 (18.2) | |
| Total preservation time 1st lung (h:min) | 5:47 (5:18–6:40) | 12:09 (9:23–13:29) | <0.001 |
| Total preservation time 2nd lung (h:min) | 7:15 (6:33–7:40) | 14:24 (12:00–16:20) | <0.001 |
| CIT 1st lung (h:min) | 5:05 (4:32–5:58) | 11:40 (9:50–13:25) | <0.001 |
| CIT 2nd lung (h:min) | 6:31 (5:34–7:28) | 13:36 (11:20–15:20) | <0.001 |
| Postoperative outcomes | |||
| PGD grade 3 at 72h (%) | 3 (12.5) | 3 (9.4) | 0.440 |
| Postoperative ECMO (%) | 4 (16.7) | 4 (12.1) | 0.626 |
| MV recipient, days (median, IQR) | 2 (1–11) | 1 (0.8–10.5) | 0.758 |
| ICU stay, days (median, IQR) | 9 (6–23) | 8 (6–18) | 0.814 |
| Hospital stay, days (median, IQR) | 45 (35–75) | 42 (36–54) | 0.297 |
| 30-d mortality (%) | 1 (4.2) | 0 | 0.237 |
| 90-d mortality (%) | 1 (4.2) | 0 | 0.237 |
| Best post-op FEV1 (median, IQR) | 2380 (1595–2740) | 2170 (2020–2680) | 0.913 |
| Acute cellular rejection (%) | 8 (34.8) | 9 (28.1) | 0.598 |
DBD: brain death donor; DCD: donor after controlled circulatory death; MV: mechanical ventilation; P/F: arterial oxygen partial pressure/fractional inspired oxygen; LTx: lung transplantation; COPD: chronic obstructive pulmonary disease; IPF: idiopathic pulmonary fibrosis; ILD: interstitial lung disease; LAS: lung allocation score; BMI: body max index; 6MWT: 6min walking test; mPAP: median pulmonary artery pressure; pTLC: predictive total lung capacity; CIT: cold ischemia time; ECMO: extracorporeal membrane oxygenation; CPB: cardiopulmonary bypass; PGD: primary graft dysfunction; FEV1: forced expiratory volume 1s.
Total preservation times (h:min) from donor cross-clamp to recipient reperfusion were statistically longer (p<0.001) in the 10°C group [1st lung: median 12:09 (IQR 9:23–13:29; minimum 5:32; maximum 17:15); 2nd lung: median 14:24 (IQR 12:00–16:20; minimum 8:20; maximum 18:40)] in comparison to the standard group [1st lung: median 5:47 (IQR 5:18–6:40; minimum 5:20; maximum 6:40); 2nd lung: median 7:15 (IQR 6:33–7:40; minimum 6:30; maximum 7:40)]. Median lung transport time (h:min) in the ice cooler was 3:15 in the 10°C group (IQR 2:30–3:55). 10°C preservation time (h:min) represents the period of intentional extension of the CIT [1st lung: median 6:50 (IQR 2:00–12:58); 2nd lung: median 8:35 (IQR 3:40–14:19)]. The CIT (h:min) for both the first and second implanted lungs were also significantly longer in the 10°C group [1st lung: median 11:40 (IQR 9:50–13:25); 2nd lung: median 13:36 (IQR 11:20–15:20)] in comparison to the standard group [1stlung: median 5:05 (IQR 4:32–5:58); 2nd lung: median 6:31 (IQR 5:34–7:28)]. The preservation times are displayed in Fig. 3a and b.
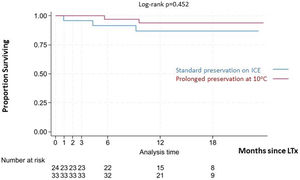
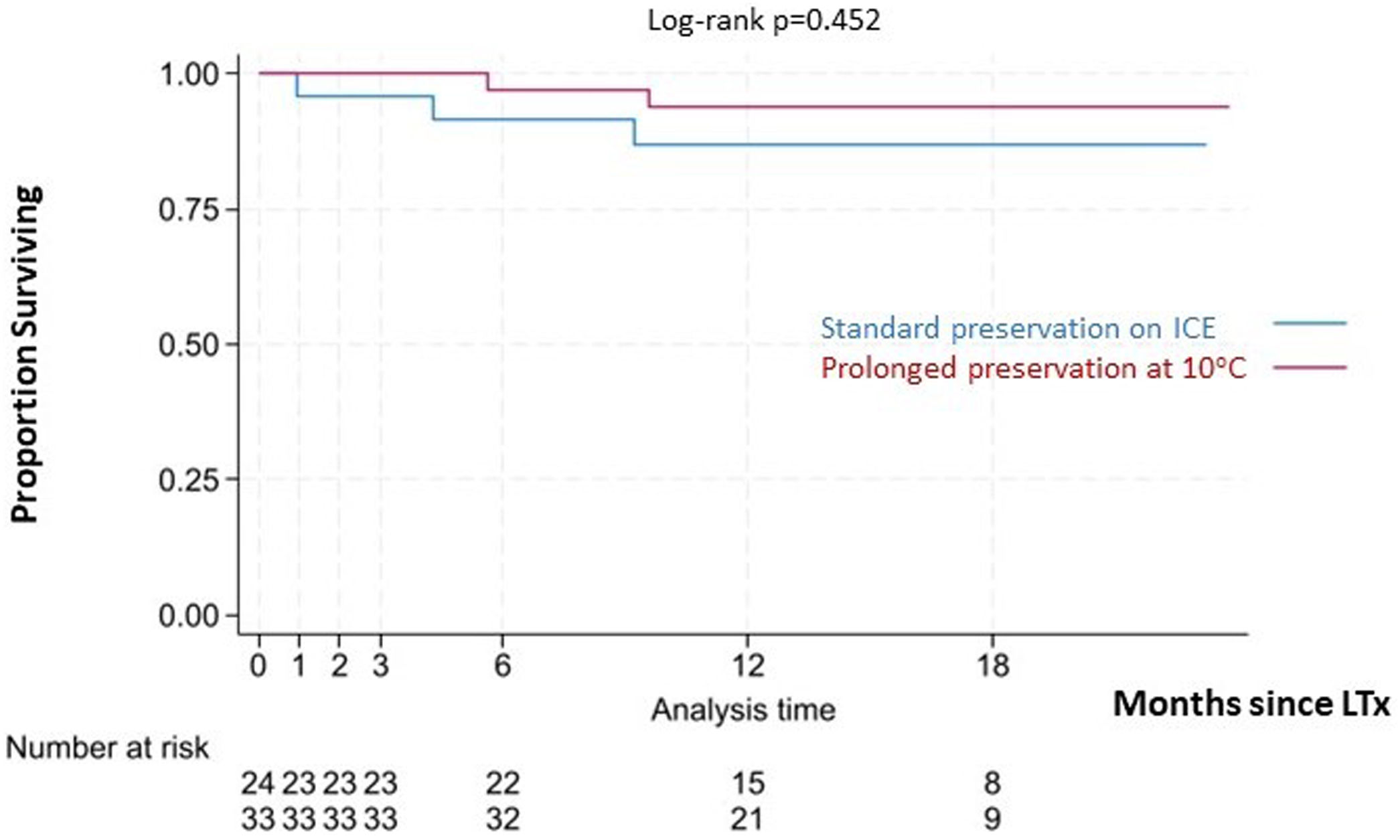
No statistically significant differences were detected regarding PGD grade 3 at 72-h (9.4% in the 10°C group vs. 12.5% in the standard group) or the need for postoperative ECMO (12.1% in the 10°C group vs. 16.7% in the standard group). The median length of MV (1 vs. 2 days), ICU stay (8 vs. 9 days) and hospital stay (42 vs. 45 days) were similar in both groups (Table 1). No deaths were observed at 30 and 90 days in the 10°C group. One patient (4.2%) from the standard preservation group passed away 28 days post-operation due to sepsis. The median follow-up time was 15.36 months (IQR 12.5–17.1). Survival outcomes for the 10°C group were similar compared to the standard group (100% vs. 95.8% at 90 days; 97% vs. 92% at 180 days, 93% vs. 87% at 1 year, respectively; log-rank p=0.363). Fig. 4 shows the Kaplan–Meier survival curves.
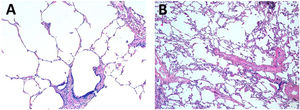
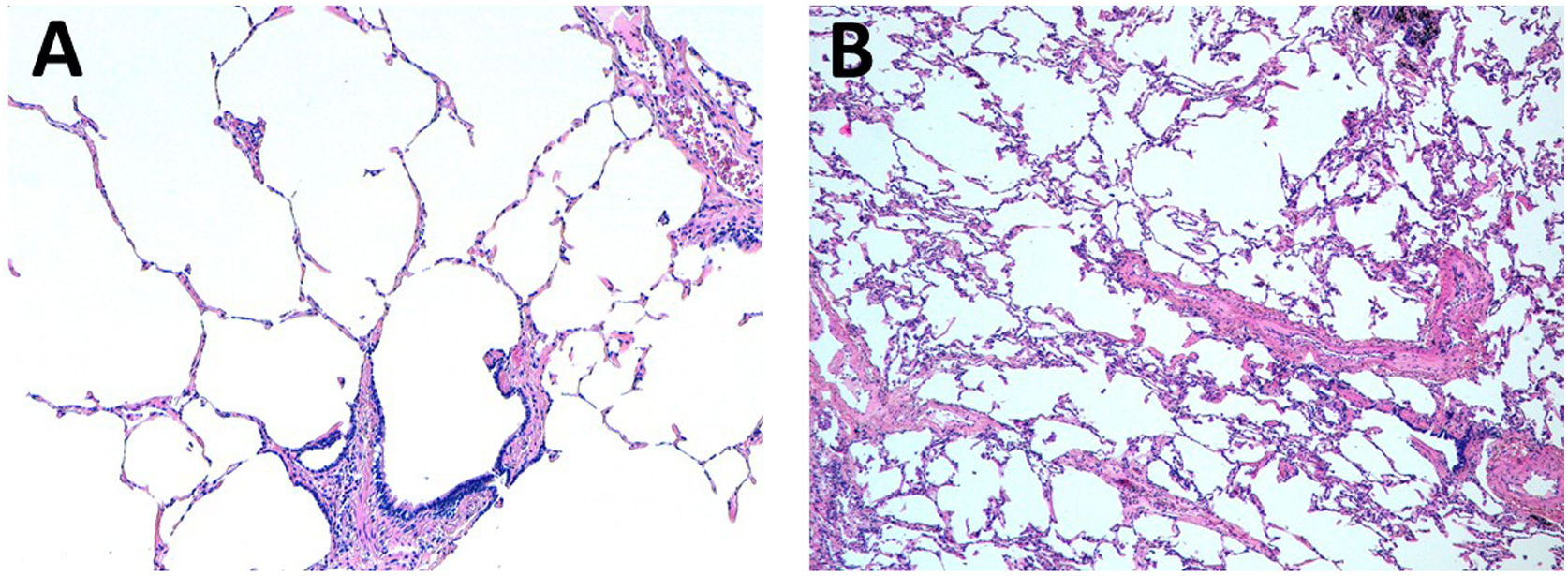
The median ALI score for postreperfusion biopsy was 1 point for the 10°C group (IQR 0–1) and 0 for the standard group (IQR (0–1). The histopathological findings of two cases carried out by different methods of preservation are illustrated in Fig. 5.
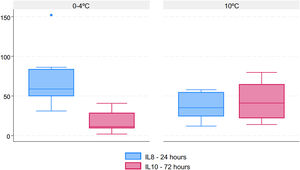
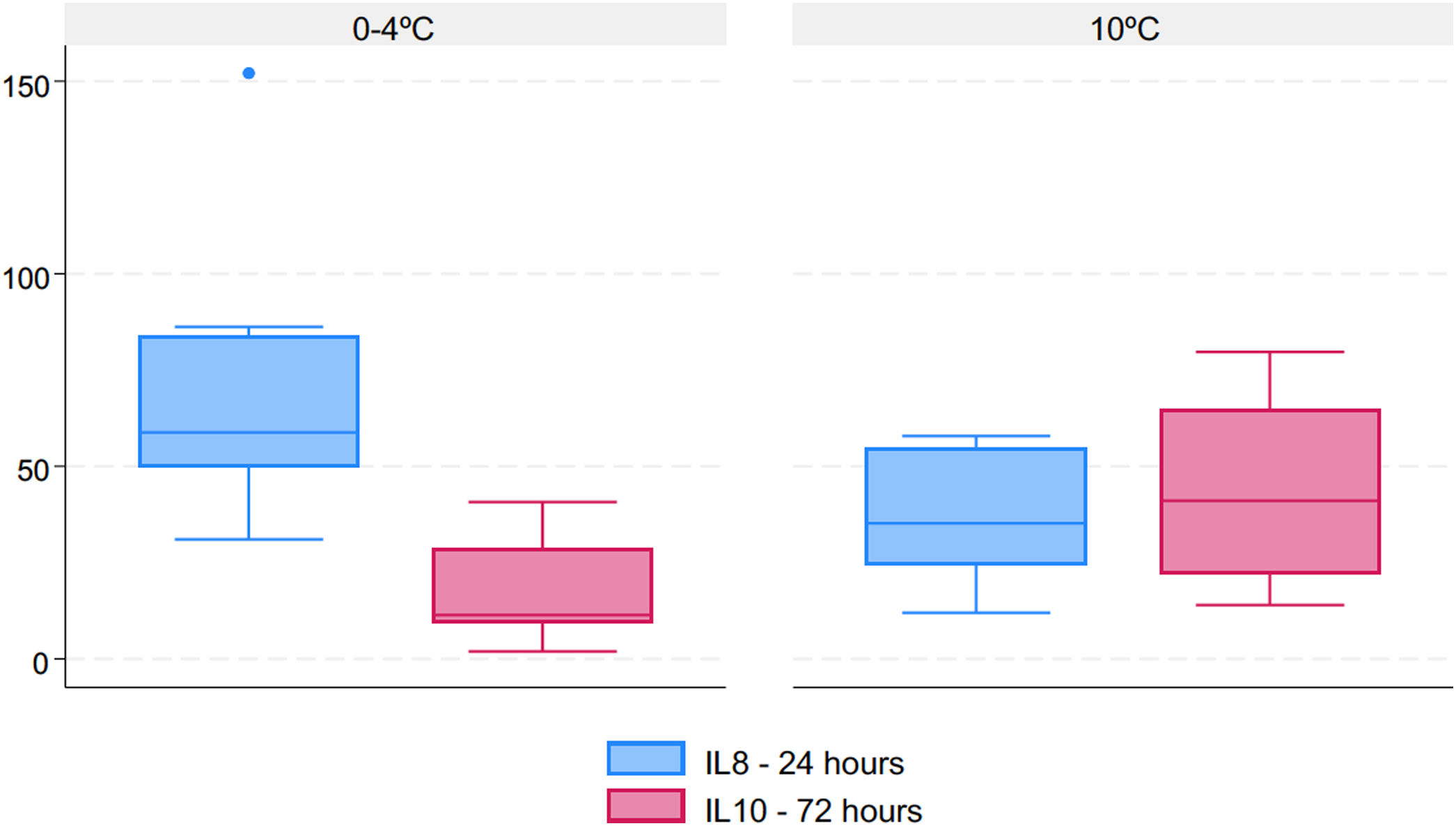
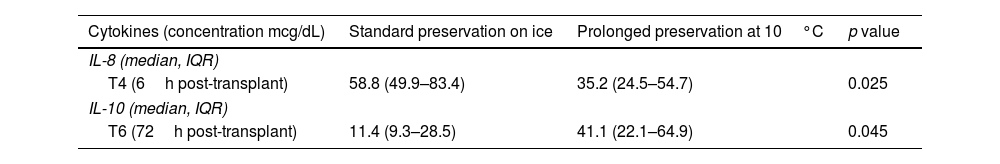
Regarding the cytokine expression profile (Table 2), there was a significantly higher IL-8 concentration 6h after transplant (T4) with standard preservation on ice (p=0.025) and a significantly increased IL-10 concentration 72h after transplant (T6) in the 10°C group (p=0.045). The graphs comparing cytokine profiles are displayed in Fig. 6.
Cytokine expression profile comparing both preservation methods.
| Cytokines (concentration mcg/dL) | Standard preservation on ice | Prolonged preservation at 10°C | p value |
|---|---|---|---|
| IL-8 (median, IQR) | |||
| T4 (6h post-transplant) | 58.8 (49.9–83.4) | 35.2 (24.5–54.7) | 0.025 |
| IL-10 (median, IQR) | |||
| T6 (72h post-transplant) | 11.4 (9.3–28.5) | 41.1 (22.1–64.9) | 0.045 |
Despite the continuous efforts on improving donor and recipient management,18 PGD remains a significant source of early morbidity and mortality.19,20 Similarly, the maximum CIT of 6–8h has traditionally been associated with the occurrence of PGD.3,21 Although recent studies suggest that current preservation techniques allow for a safe extension of the CIT without increasing the risk of developing PGD grade 3,22–24 transplant surgeons are normally conservative while using the standard preservation approach. Therefore, despite the available evidence, the maximum safe CITs are still uncertain. Following the new evidence on the safety and feasibility of extending the CIT to the temperature of 10°C,7,25,26 our results support the fact that prolonged preservation at 10°C provides comparable early outcomes to the standard method, specifically in terms of the incidence of PGD grade 3 at 72-h. In this study, by extending the preservation times at a temperature of 10°C we did not observe a negative impact on donor lung histopathology but we saw a superior inflammatory profile.
Incidence of severe PGD at 72-h was the primary endpoint of this study based on evidence suggesting that it has a strong impact on early and late outcomes19,20 and this is influenced by organ preservation strategies. The incidence of PGD grade 3 at 72-h in the study group (9.4%) did not differ from the control group, despite total preservation times of up to 18h. Secondary endpoints, such as length of MV, hospital stay, and ICU stay were similar in both groups. All patients in the study group survived to the 30th and 90th day post-LTx. Clinical outcomes of this study are consistent with those recently published by the multicenter clinical trial on 10°C preservation.9
The impact of the preservation strategy on the post-LTx cytokine expression profile has recently been analyzed. An animal study concluded that extending the CIT from 6 to 12h using the standard preservation method on ice did not modify the expression of pro-inflammatory cytokines (IL-1β, IL-6, IFN-gamma).22 Additionally, the metabolomic benefits of 36-h preservation at 10°C has been described in experimental animal models showing significantly lower concentrations of IL-1β and IL-8 in the EVLP perfusate for lungs stored at 10°C versus those stored on ice.7 Moreover, in the setting of injured porcine lungs, 12-h preservation at 10°C showed reduced histological damage and lower tissue concentrations of IL-1β, in comparison to those immediately implanted after standard preservation on ice.26 Our results are in keeping with the previous studies, revealing a significant increase in the proinflammatory profile using the standard approach (IL-8 serum concentration 6h after LTx) and a protective anti-inflammatory benefit when preserved under 10°C (IL-10 serum concentration 72h after LTx). While IL-8 has been shown to be associated with early onset inflammation and PGD, IL-10 might have positive effects on allograft outcomes by inhibiting inflammatory responses.27 In fact, treating injured human donor lungs with IL-10 may enhance lung function, potentially making injured lungs suitable for LTx.28 These findings in the cytokine profile expression are aligned with other markers such as a low ALI score and less severe PGD incidence, pointing out that there is no significant inflammatory insult when prolonging the CIT using 10°C preservation.
An additional important issue is the observation made in several studies that surgical procedures performed at nighttime appear to be associated with a higher risk of surgical complications.29,30 Specifically, in the context of transplantation, some authors have linked the time of implantation to early morbidity and mortality outcomes.31 More specifically, in the field of LTx, Yang et al. suggested that LTx performed at nighttime, between 6:00p.m. and 6:00a.m. were associated with a higher risk of major adverse events in the postoperative period.32 Exploring the concept that performing procedures during daytime may result in a safer outcome is what has motivated these studies to intentionally extend preservation times under 10°C to achieve a semi-elective procedure. However, it is not only to avoid nighttime procedures; there are many other reasons behind this decision, such us delaying complex cases to the next morning. For instance, recipients in an urgent status with ECMO as a bridge or for whom long dissection is expected due to previous surgeries could benefit from a morning OR start time when the majority of hospital personnel are present.25 Prolonged preservation at 10°C also facilitate pre-transplant treatments in pre-sensitized recipients and enabled us to perform intraoperative plasmapheresis at our site, which is only logistically possible in the mornings on working days. Another logistical benefit has been the opportunity to accept two or more donors at the same time and delaying one of the cases in order to spread the workload between transplant teams.11 In fact, waiting for a frozen section to rule out a malignancy in donors with a suspicious nodule has classically been a reason for declining a graft in our institution due to a possible prolonged CIT before the 10°C preservation strategy became available. Among the demand and scarcity of suitable donors, another potential opportunity for our program could be assessing donor lungs in distant regions and improving organ sharing thanks to 10°C preservation. We strongly believe that the aforementioned logistical benefits have a critical impact on LTx programs, particularly in those programs located in medium-sized hospitals, as it could increase flexibility with regard to the scheduling of LTx operations and lead to a greater focus on patient safety.
This study has several limitations, mainly the relatively small number of cases. However, it provides both contemporary and comparable groups of patients showing promising outcomes and increases knowledge surrounding the novel strategy of 10°C preservation. Nevertheless, conducting a randomized trial comparing 10°C to standard cold storage on ice would help to definitively clarify whether preservation at 10°C leads to equal or better outcomes when intentionally extending CIT. Transporting the lungs on ice before being transferred to the 10°C device represents another limitation of our study, diminishing the hours the grafts could be beneficially preserved at 10°C. Furthermore, the inability to constantly monitor the temperature during both preservation strategies might be another weakness of this study. In contrast, this study has several strengths, such as having performed a non-matched analysis between groups, which provides a raw analysis but a close approximation of what happens in real life.
ConclusionIn conclusion, our study reinforces that LTx after extended preservation at 10°C yields a low incidence of PGD and comparable early outcomes to the standard method with significantly longer preservation times. Extending preservation times by up to 18h at a temperature of 10°C greatly improved logistics at our site and has the potential to revolutionize the field of LTx.
Statement of IRB/Ethics board approvalThis study was approved by the Ethics Committee of our institution, in accordance with the Declaration of Helsinki (Project Code: 118/21, 29 September 2021).
Statement of informed consentAll patients enrolled in the study provided a signed informed consent.
Funding statementThis study has been awarded grants by the following scientific societies: Fundación Mutua Madrileña, Fundación Neumomadrid, Fundación SEPAR RESPIRA, Sociedad Madrileña de Trasplante, Fundación Sociedad Española de Cirugía Torácica.
Conflict of interestThe authors declare that they have not financial interests that relate the research described in this paper.
We acknowledge the dedicated work of Transplant Coordinators, Anesthesiology, ICU, Pneumology, Pathology, Immunology departments, which have contributed to this study. The authors acknowledge the critical review from Marcelo Cypel and Konrad Hoetzenecker.




![Transport, cold ischemia and preservation times. (a) Preservation times for both groups. Median times for the first and second lung respectively [IQR]. (b) Box plot showing the different preservation times. Transport, cold ischemia and preservation times. (a) Preservation times for both groups. Median times for the first and second lung respectively [IQR]. (b) Box plot showing the different preservation times.](https://static.elsevier.es/multimedia/03002896/unassign/S0300289624000814/v1_202404220407/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w98FxLWLw1xoW2PaQDYY7RZU=)