We examined fifteen years trends (2001–2015) in the use of non-invasive ventilation (NIV), invasive mechanical ventilation (IMV) or both (NIV+IMV) among patients hospitalized for community acquired pneumonia (CAP). We also analyzed trends overtime and the influence of patient factors in the in-hospital mortality (IHM) after receiving NIV, IMV or NIV+IMV.
MethodsObservational retrospective epidemiological study. Our data source was the Spanish National Hospital Discharge Database.
ResultsOver a total of 1,486,240 hospitalized patients with CAP, we identified 56,158 who had received ventilator support in Spain over the study period. Of them, 54.82% received NIV, 37.04% IMV and 8.14% both procedures. The use of NIV and NIV+IMV increased significantly (p<0.001) over time (from 0.91 to 12.84 per 100.000 inhabitant and from 0.23 to 1.19 per 100.000 inhabitants, respectively), while the IMV utilization decreased (from 3.55 to 2.79 per 100,000 inhabitants; p<0.001). Patients receiving NIV were the oldest and had the highest mean value in the Charlson comorbidity index (CCI) score and readmission rate. Patients who received only IMV had the highest IHM. Factors associated with IHM for all groups analyzed included age, comorbidities and readmission. IHM decreased significantly over time in patients with CAP who received NIV, IMV and NIV+IMV.
ConclusionsWe found an increase in NIV use and a decline in IMV utilization in patients hospitalized for CAP over the study period. Patients receiving NIV were the oldest and had the highest CCI score and readmission rate. IHM decreased significantly over time in patients with CAP who received NIV, IMV and NIV+IMV.
Estudiamos las tendencias a lo largo de 15 años (2001-2015) en el uso de la ventilación no invasiva (VNI), la ventilación mecánica invasiva (VMI) o ambas (VNI+VMI) en los pacientes hospitalizados por neumonía adquirida en la comunidad (NAC). También analizamos las tendencias en el tiempo y la influencia de los factores del paciente en la mortalidad hospitalaria (MH) después de recibir VNI, VMI o VNI+VMI.
MétodosEstudio epidemiológico retrospectivo observacional. Nuestra fuente de datos fue el Registro de Altas de los Hospitales (CMBD) del Sistema Nacional de Salud.
ResultadosEn un total de 1.486.240 pacientes hospitalizados por NAC, identificamos a 56.158 que habían recibido soporte ventilatorio en España durante el período a estudio. De ellos, el 54,82% recibió VNI, el 37,04% VMI y el 8,14% ambos procedimientos. El uso de VNI y VNI+VMI aumentó significativamente (p<0,001) con el tiempo (de 0,91 a 12,84 por habitante y de 0,23 a 1,19 por cada 100.000 habitantes, respectivamente), mientras que la utilización de la VMI disminuyó (de 3,55 a 2,79 por cada 100.000 habitantes; p<0,001). Los pacientes que recibieron VNI fueron los más ancianos y presentaban el valor medio más alto de puntuación en el índice de comorbilidad de Charlson (CCI, por sus siglas en inglés) y en la tasa de reingreso. Los pacientes que recibieron solo VMI presentaron la MH más alta. Los factores asociados a la MH para todos los grupos analizados incluyeron la edad, las comorbilidades y el reingreso. La MH disminuyó significativamente con el tiempo en los pacientes con NAC que recibieron VNI, VMI y VNI+VMI.
ConclusionesEncontramos un aumento en el uso de VNI y una disminución en la utilización de VMI en pacientes hospitalizados por NAC durante el período a estudio. Los pacientes que recibieron VNI fueron los más ancianos y tenían la puntuación más alta en el CCI y la tasa de reingreso más elevada. La MH disminuyó significativamente con el tiempo en los pacientes con NAC que recibieron VNI, VMI y VNI+VMI.
Community acquired pneumonia (CAP) is a leading cause of emergency department visits and hospitalizations worldwide.1 One of the most relevant complications of this disease is acute respiratory failure, which can occur in 58–87% of patients with severe CAP and is associated with a high mortality rate. Its presence may be used to assess CAP severity and the need for hospitalization.2–4
When patients with CAP develop severe respiratory failure despite antibiotics and other supportive therapies, ventilatory support can be required.5 Both non-invasive ventilation (NIV) and invasive mechanical ventilation (IMV) are standard approaches to the treatment of this complication.6 However, the impact of the initial ventilatory mode on clinical outcomes is not well understood nowadays. In fact, there is a paucity of evidence regarding criteria for selection to patients to receive NIV treatment.6 In addition, the few preexisting randomized studies which compare NIV to IMV have controversial results.2,7,8 Probably discordance between them is due to differences in the study design, the severity of participants or exclusion criteria used.9
Given the lack of evidence, it remains unclear if NIV is a good therapeutic option for patients with CAP presenting with severe acute respiratory failure.10 Currently clinical guidelines recommend caution in using this mode of ventilatory support in such circunstances.11,12 Nevertheless, NIV is frequently used in emergency departments and intensive care units (ICUs) to treat patients with severe pneumonia, in order to avoid intubation.13–15
Using data from the population-based Spanish National Hospital Discharge Database (SNHDD), we examined fifteen-year trends (2001–2015) in the incidence of ventilatory support with NIV, IMV or both (NIV+IMV) among patients hospitalized with CAP. Secondly, we assessed the changes overtime in the prevalence and the influence of patient's characteristics on receiving NIV, IMV or NIV+IMV. Finally, we analyzed the trends and variables associated with in-hospital mortality (IHM) after receiving NIV, IMV or NIV+IMV in patients suffering CAP.
MethodsWe conducted an observational retrospective epidemiological study. Our data source was the SNHDD. This database contains de-identified clinical and resource utilization data of over 95% of the hospital discharges per year in Spain.16
The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) system is used for coding. Additionally, hospital outcome variables, such as length of hospital stay (LOHS), readmission, and IHM, are collected by the SNHDD.
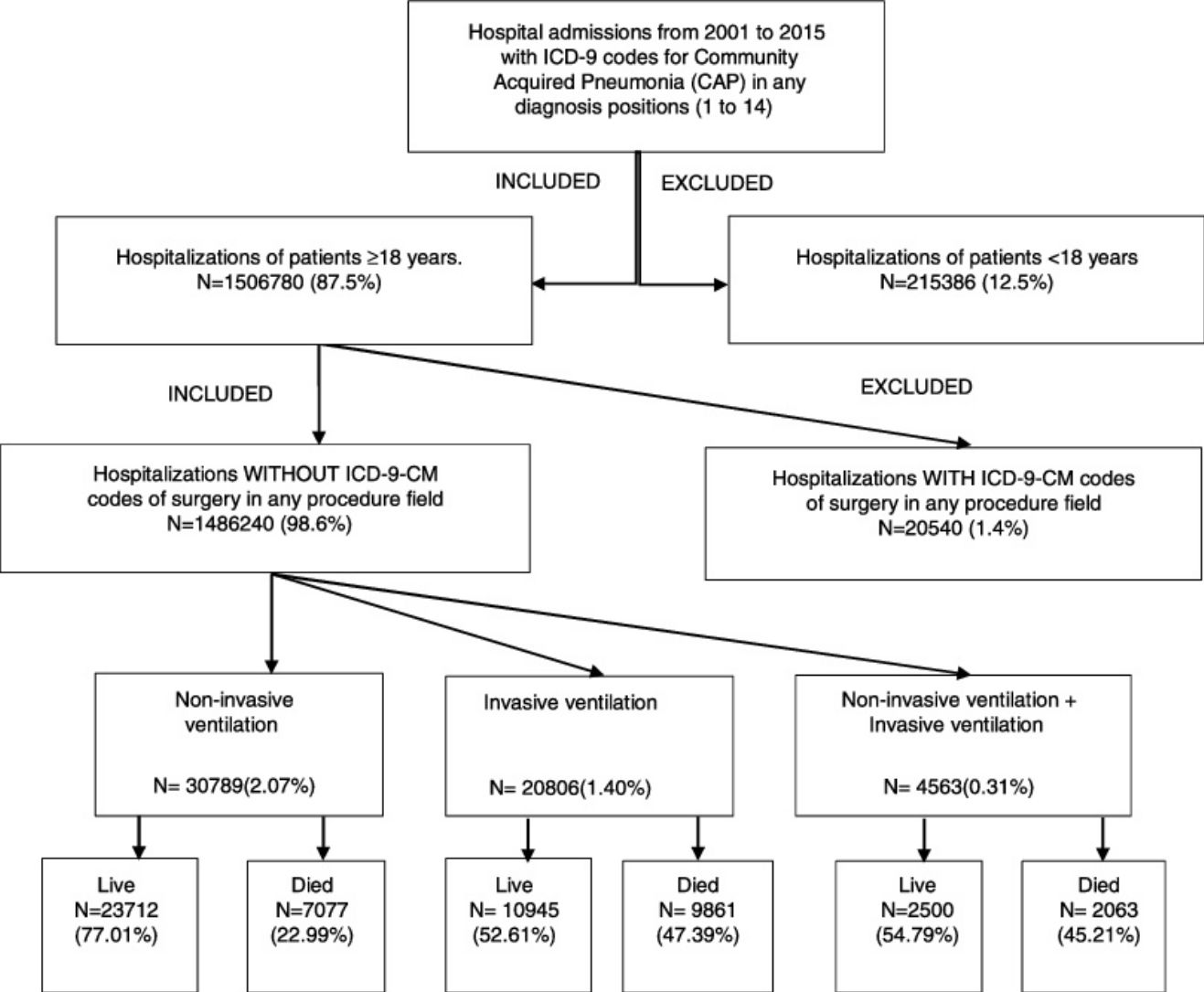
For study purposes and, following the method described by Guevara et al.,17 we included hospitalizations of patients ≥18 years of age with a principal discharge diagnosis of CAP (ICD-9-CM codes: 480–488, 507.0–507.8) or a principal diagnosis of sepsis (ICD-9-CM codes: 038,995.92,995.91,785.52) or respiratory failure (ICD-9-CM codes: 518.81,518.82,518.84,799.1) or meningitis (ICD-9-CM codes: 322.xx) or emphysema (ICD-9-CM codes: 510.0,510.9) or bacteremia (ICD-9-CM code: 790.7) paired with a secondary diagnosis of CAP who were discharged between 01/01/2001 and 31/12/2015.
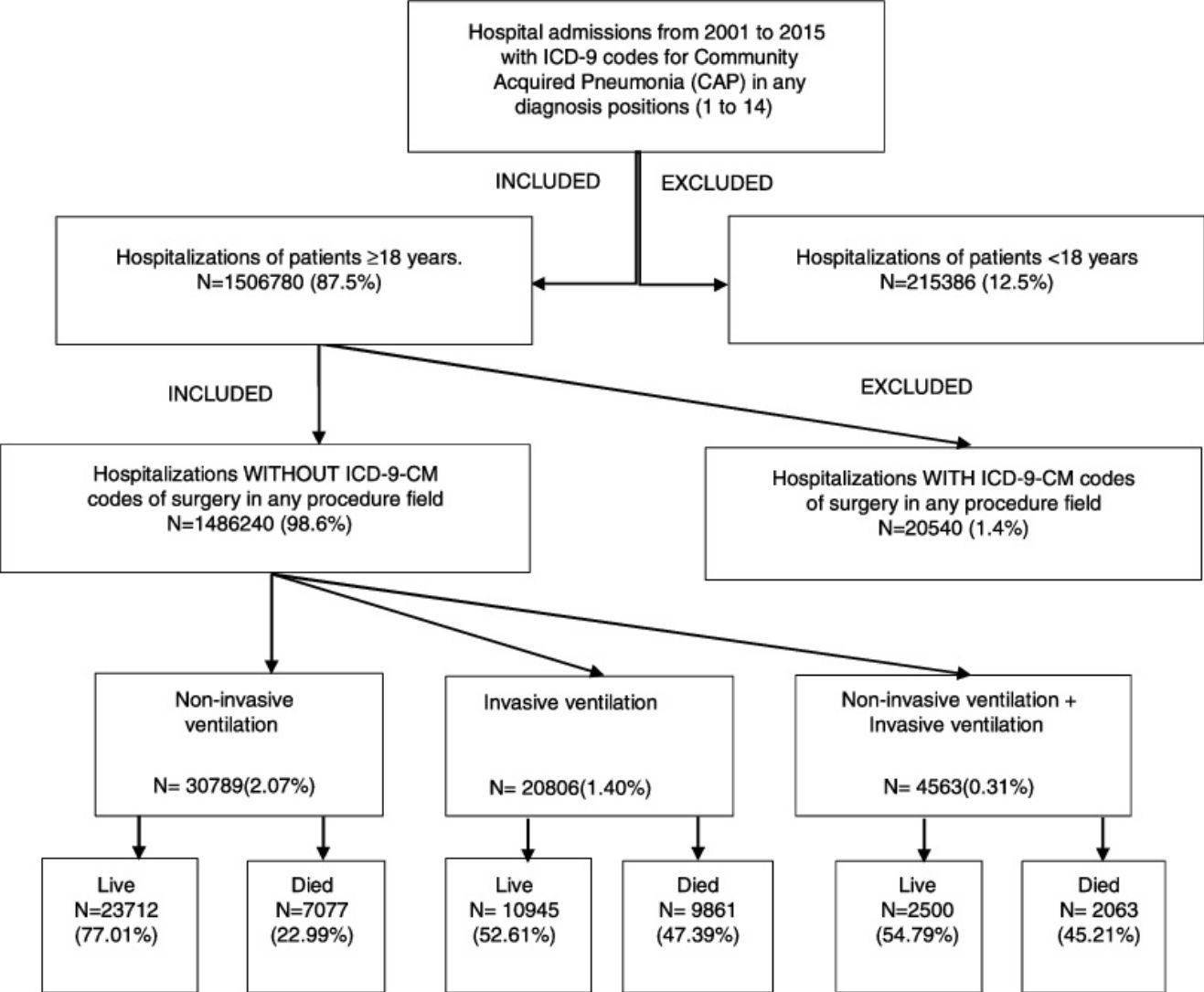
We excluded all hospitalizations with an ICD-9-CM code for ventilator associated pneumonia (997.31) in any diagnosis field. Furthermore, we excluded all hospitalizations with an ICD-9-CM code of surgery as described Metha et al.18 in any procedure field in the SNHDD. A Flow Chart for patient selection is shown in the Graphic Summary
We considered a patient to have received NIV or IMV during the admission if there was an ICD-9-CM procedure code for NIV (93.90 or 93.91) or IMV (96.70, 96.71, or 96.72) in any procedure field. The codes for NIV include both Non-invasive Bi-level Ventilation and Continuous Positive Airway Pressure (CPAP).
We defined three cohorts of patients: those who received only NIV, those who received only IMV and those who received NIV+IMV. The database does not allow establishing the temporal sequence of treatments, and the NIV+IMV group thus encompassed both NIV succeeded by IMV and IMV succeeded by NIV.
The main outcome variable of our investigation is the incidence in the use of NIV, IMV or NIV+IMV in patients with CAP. Secondarily, we assessed the IHM in patients with CAP who received NIV, IMV or NIV+IMV.
For each hospital admission, we recorded covariates such as demographic information (age and sex), diagnosed comorbidities, therapeutic procedures and hospital variables (readmission, LOHS and IHM).
To assess the burden of comorbidity, all conditions included in the Charlson Comorbidity Index (CCI) coded in any diagnosis position (1–14) in the discharge report were identifiedl.19 The ICD-9 codes used to identify the conditions of the CCI are shown in Supplementary Table 1. We analyzed conditions of the CCI individually and as a sum.
We analyzed pneumonia pathogens documented using the following ICD-9-CM codes: 481 for Streptococcus pneumoniae; 482.84 for Legionella; 482.41 and 482.42 for Staphylococcus aureus; 482.2 for Haemophilus influenzae; and 482.1 for Pseudomonas aeruginosa. These were the five most frequently identified pathogens.
The diagnosis of aspiration pneumonia (ICD-9-CM code 507.0–507.8) during the hospitalization was analyzed.
We estimated the proportion of readmission (patients that had been discharged from the hospital within the previous 30days), the mean of LOHS and IHM. IHM is defined by the proportion of patients who died during admission for each year of study.
Statistical methodsTo estimate the incidence of hospital admission with NIV, IMV and NIV+IMV in patients with CAP, we divided the number of these procedures each year by the corresponding Spanish population for that same year.20
To assess changes in the incidence over time, Joinpoint Trend Analysis Software Version 4.7.0.0 was used (Statistical Research and Applications Branch, National Cancer Institute, Bethesda, USA). This software enabled us to identify a statistically significant change in a trend and to calculate the annual percentage change (APC) after each time point of change.21
Data are expressed as means (SD) for continuous variables and frequencies and proportions for categorical data. We compared differences in continuous variables using Student's t-test, the Mann–Whitney U test, ANOVA, and the Kruskal–Wallis test, as appropriate. Categorical variables were compared using Chi-square tests.
We performed multivariable logistic regression models to assess the time trend and to identify factors associated with IHM for each ventilator support type. Variables included in the models were those that yielded a significant association with IHM in the bivariate analysis. Odds ratios with 95% confidence intervals are shown.
All statistical tests were conducted with an α value of 0.05 except for tests with multiple pairwise comparisons where a Bonferroni correction was used.
All statistical analyses were performed using Stata Software 11.0 (StataCorp LP, College Station, Texas, USA).
Ethical aspectsRetrospective use of de-identified registry data does not require ethical approval or informed consent according to Spanish legislation.
ResultsPatient and hospital characteristicsA total of 1,486,240 hospitalizations of patients aged 18 years or older with CAP in Spain (2001–2015) were included.
An episode of CAP was identified more frequently among men (60.52%) than women and the mean age at admission was 73.1 years (SD 16.43 years). The percentage of males affected decreased significantly (p<0.001) over time (63.48% in 2001/03 vs. 58.32% in 2013/15) and the mean age increased significantly over time (Table 1).
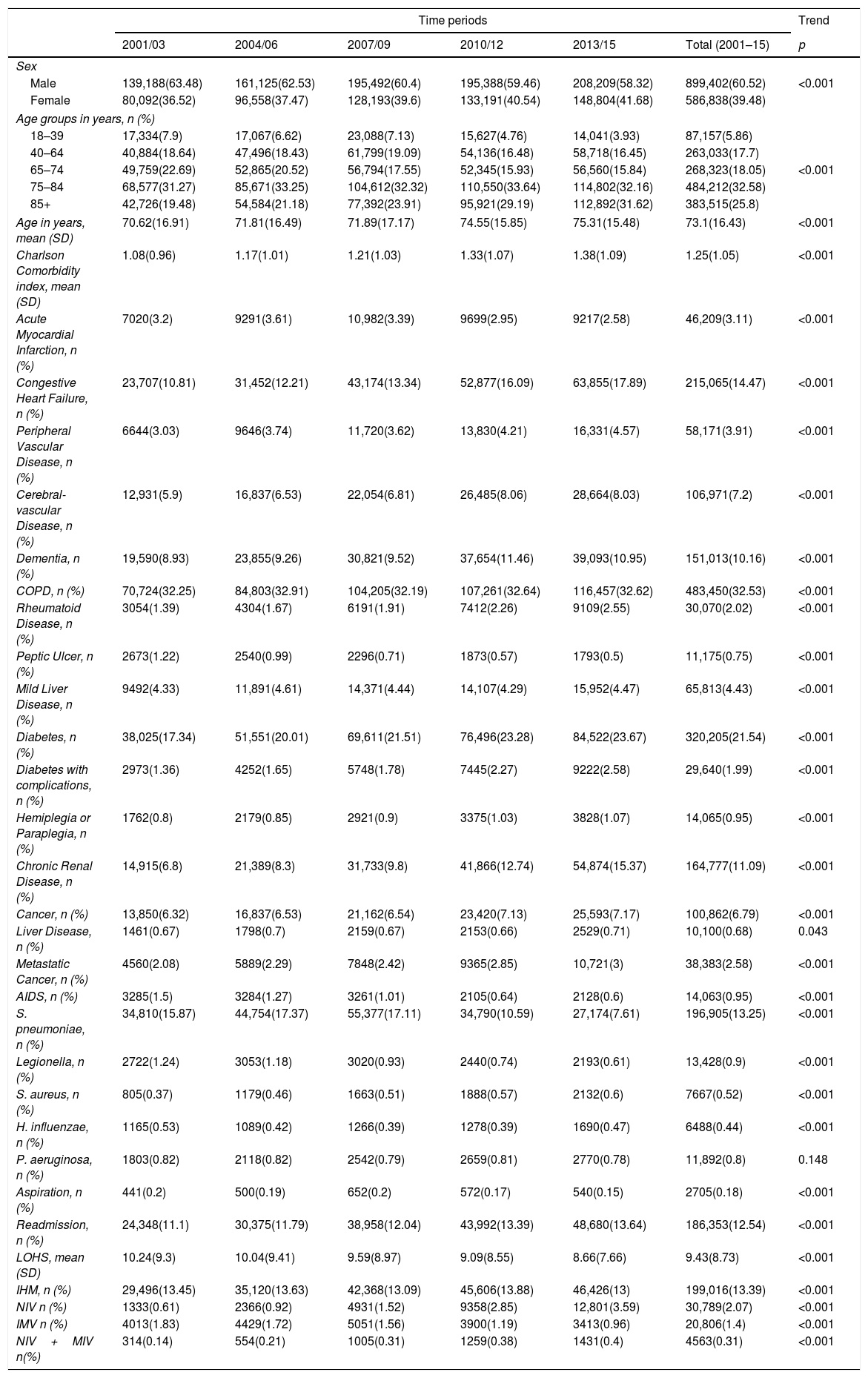
Trends in the characteristics and use of mechanical ventilation of hospital admission with community acquired pneumonia in Spain from 2001 to 2015 (Spanish National Hospital Discharge Database).
| Time periods | Trend | ||||||
|---|---|---|---|---|---|---|---|
| 2001/03 | 2004/06 | 2007/09 | 2010/12 | 2013/15 | Total (2001–15) | p | |
| Sex | |||||||
| Male | 139,188(63.48) | 161,125(62.53) | 195,492(60.4) | 195,388(59.46) | 208,209(58.32) | 899,402(60.52) | <0.001 |
| Female | 80,092(36.52) | 96,558(37.47) | 128,193(39.6) | 133,191(40.54) | 148,804(41.68) | 586,838(39.48) | |
| Age groups in years, n (%) | |||||||
| 18–39 | 17,334(7.9) | 17,067(6.62) | 23,088(7.13) | 15,627(4.76) | 14,041(3.93) | 87,157(5.86) | |
| 40–64 | 40,884(18.64) | 47,496(18.43) | 61,799(19.09) | 54,136(16.48) | 58,718(16.45) | 263,033(17.7) | |
| 65–74 | 49,759(22.69) | 52,865(20.52) | 56,794(17.55) | 52,345(15.93) | 56,560(15.84) | 268,323(18.05) | <0.001 |
| 75–84 | 68,577(31.27) | 85,671(33.25) | 104,612(32.32) | 110,550(33.64) | 114,802(32.16) | 484,212(32.58) | |
| 85+ | 42,726(19.48) | 54,584(21.18) | 77,392(23.91) | 95,921(29.19) | 112,892(31.62) | 383,515(25.8) | |
| Age in years, mean (SD) | 70.62(16.91) | 71.81(16.49) | 71.89(17.17) | 74.55(15.85) | 75.31(15.48) | 73.1(16.43) | <0.001 |
| Charlson Comorbidity index, mean (SD) | 1.08(0.96) | 1.17(1.01) | 1.21(1.03) | 1.33(1.07) | 1.38(1.09) | 1.25(1.05) | <0.001 |
| Acute Myocardial Infarction, n (%) | 7020(3.2) | 9291(3.61) | 10,982(3.39) | 9699(2.95) | 9217(2.58) | 46,209(3.11) | <0.001 |
| Congestive Heart Failure, n (%) | 23,707(10.81) | 31,452(12.21) | 43,174(13.34) | 52,877(16.09) | 63,855(17.89) | 215,065(14.47) | <0.001 |
| Peripheral Vascular Disease, n (%) | 6644(3.03) | 9646(3.74) | 11,720(3.62) | 13,830(4.21) | 16,331(4.57) | 58,171(3.91) | <0.001 |
| Cerebral-vascular Disease, n (%) | 12,931(5.9) | 16,837(6.53) | 22,054(6.81) | 26,485(8.06) | 28,664(8.03) | 106,971(7.2) | <0.001 |
| Dementia, n (%) | 19,590(8.93) | 23,855(9.26) | 30,821(9.52) | 37,654(11.46) | 39,093(10.95) | 151,013(10.16) | <0.001 |
| COPD, n (%) | 70,724(32.25) | 84,803(32.91) | 104,205(32.19) | 107,261(32.64) | 116,457(32.62) | 483,450(32.53) | <0.001 |
| Rheumatoid Disease, n (%) | 3054(1.39) | 4304(1.67) | 6191(1.91) | 7412(2.26) | 9109(2.55) | 30,070(2.02) | <0.001 |
| Peptic Ulcer, n (%) | 2673(1.22) | 2540(0.99) | 2296(0.71) | 1873(0.57) | 1793(0.5) | 11,175(0.75) | <0.001 |
| Mild Liver Disease, n (%) | 9492(4.33) | 11,891(4.61) | 14,371(4.44) | 14,107(4.29) | 15,952(4.47) | 65,813(4.43) | <0.001 |
| Diabetes, n (%) | 38,025(17.34) | 51,551(20.01) | 69,611(21.51) | 76,496(23.28) | 84,522(23.67) | 320,205(21.54) | <0.001 |
| Diabetes with complications, n (%) | 2973(1.36) | 4252(1.65) | 5748(1.78) | 7445(2.27) | 9222(2.58) | 29,640(1.99) | <0.001 |
| Hemiplegia or Paraplegia, n (%) | 1762(0.8) | 2179(0.85) | 2921(0.9) | 3375(1.03) | 3828(1.07) | 14,065(0.95) | <0.001 |
| Chronic Renal Disease, n (%) | 14,915(6.8) | 21,389(8.3) | 31,733(9.8) | 41,866(12.74) | 54,874(15.37) | 164,777(11.09) | <0.001 |
| Cancer, n (%) | 13,850(6.32) | 16,837(6.53) | 21,162(6.54) | 23,420(7.13) | 25,593(7.17) | 100,862(6.79) | <0.001 |
| Liver Disease, n (%) | 1461(0.67) | 1798(0.7) | 2159(0.67) | 2153(0.66) | 2529(0.71) | 10,100(0.68) | 0.043 |
| Metastatic Cancer, n (%) | 4560(2.08) | 5889(2.29) | 7848(2.42) | 9365(2.85) | 10,721(3) | 38,383(2.58) | <0.001 |
| AIDS, n (%) | 3285(1.5) | 3284(1.27) | 3261(1.01) | 2105(0.64) | 2128(0.6) | 14,063(0.95) | <0.001 |
| S. pneumoniae, n (%) | 34,810(15.87) | 44,754(17.37) | 55,377(17.11) | 34,790(10.59) | 27,174(7.61) | 196,905(13.25) | <0.001 |
| Legionella, n (%) | 2722(1.24) | 3053(1.18) | 3020(0.93) | 2440(0.74) | 2193(0.61) | 13,428(0.9) | <0.001 |
| S. aureus, n (%) | 805(0.37) | 1179(0.46) | 1663(0.51) | 1888(0.57) | 2132(0.6) | 7667(0.52) | <0.001 |
| H. influenzae, n (%) | 1165(0.53) | 1089(0.42) | 1266(0.39) | 1278(0.39) | 1690(0.47) | 6488(0.44) | <0.001 |
| P. aeruginosa, n (%) | 1803(0.82) | 2118(0.82) | 2542(0.79) | 2659(0.81) | 2770(0.78) | 11,892(0.8) | 0.148 |
| Aspiration, n (%) | 441(0.2) | 500(0.19) | 652(0.2) | 572(0.17) | 540(0.15) | 2705(0.18) | <0.001 |
| Readmission, n (%) | 24,348(11.1) | 30,375(11.79) | 38,958(12.04) | 43,992(13.39) | 48,680(13.64) | 186,353(12.54) | <0.001 |
| LOHS, mean (SD) | 10.24(9.3) | 10.04(9.41) | 9.59(8.97) | 9.09(8.55) | 8.66(7.66) | 9.43(8.73) | <0.001 |
| IHM, n (%) | 29,496(13.45) | 35,120(13.63) | 42,368(13.09) | 45,606(13.88) | 46,426(13) | 199,016(13.39) | <0.001 |
| NIV n (%) | 1333(0.61) | 2366(0.92) | 4931(1.52) | 9358(2.85) | 12,801(3.59) | 30,789(2.07) | <0.001 |
| IMV n (%) | 4013(1.83) | 4429(1.72) | 5051(1.56) | 3900(1.19) | 3413(0.96) | 20,806(1.4) | <0.001 |
| NIV+MIV n(%) | 314(0.14) | 554(0.21) | 1005(0.31) | 1259(0.38) | 1431(0.4) | 4563(0.31) | <0.001 |
COPD: chronic obstructive pulmonary disease. LOHS: length of hospital stay. IHM: in-hospital mortality. NIV: non-invasive mechanical ventilation. IMV: invasive mechanical ventilation.
Trend. Showing p value for trend assessed using logistic regression adjusted by age and sex.
The mean CCI was 1.25 (SD 1.05) and the most frequent comorbidities were as follows: chronic obstructive pulmonary disease (COPD) (32.53%), diabetes (21.54%) and congestive heart failure (14.47%). Mean CCI increased significantly over time (Table 1).
Of the pathogens analyzed the most commonly found was S. pneumoniae (13.25%). All other pathogens were found in under 1% of patients. S. pneumoniae, Legionella and H. influenzae decreased over time. However, we detected a significant increase of S. aureus over the study period (Table 1).
The proportion of aspiration pneumonia has shown a significantly decrease over the study period, from 0.2% to 0.15%; p<0.001.
Overall mean LOHS was 9.43 days and it decreased significantly from 10.24 days in 2001/03 to 8.66 days in 2013/15. Readmission increased during the study. The increase was from 11.1% to 13.64%. Over the entire period de IHM was 13.39%. Crude IHM decreased significantly over time, from 13.75% in 2001/03 to 13% in 2013/15 (Table 1).
Time trends in the use of ventilator supportAccording to the SNHDD, 56,158 patients with CAP received ventilator support in Spain from 2001 to 2015. Of them, 54.82% received only NIV; 37.04%, only IMV; and 8.14%, both procedures.
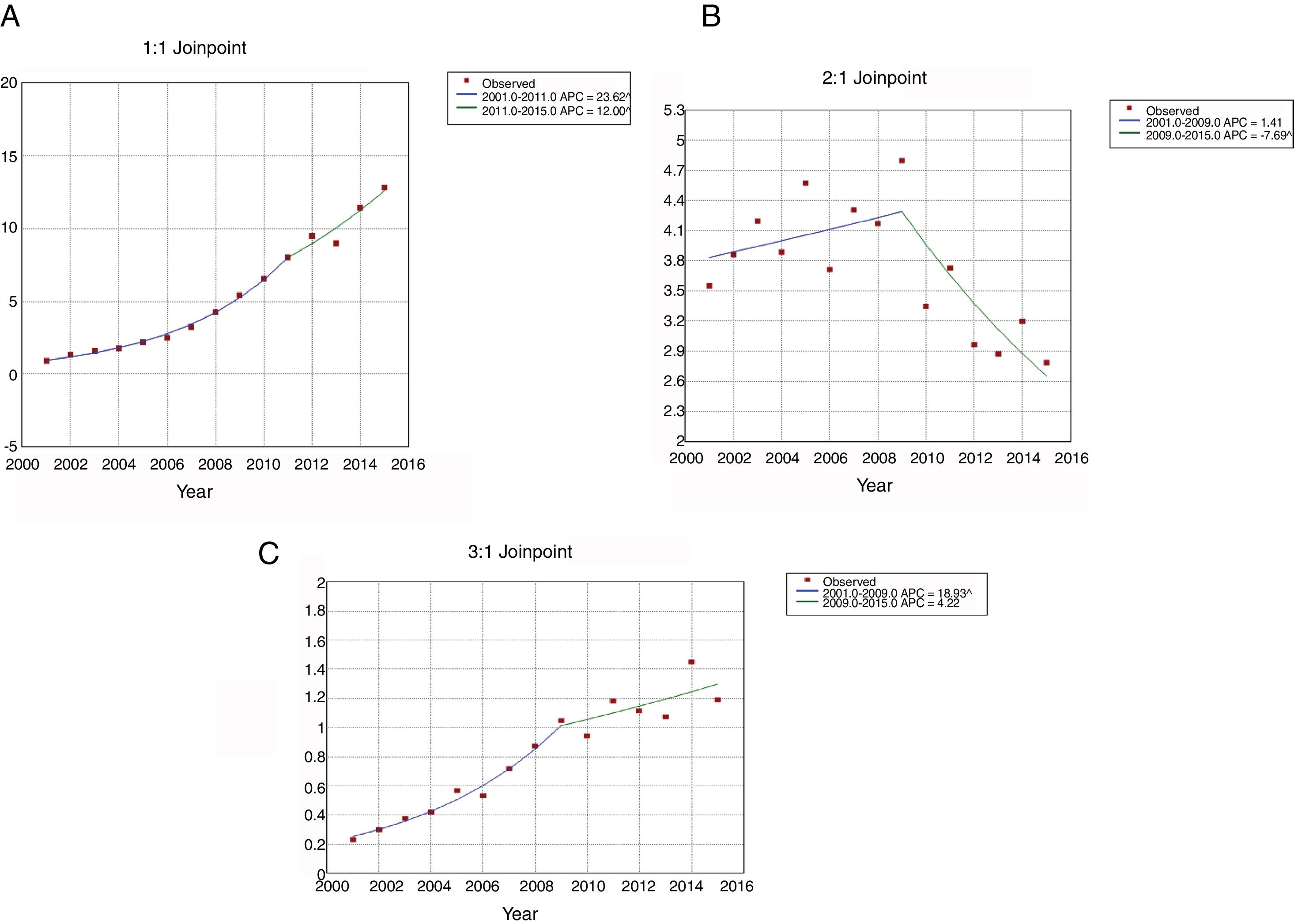
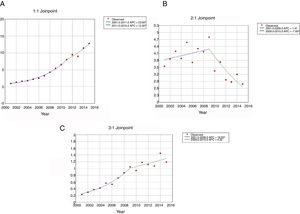
The use of NIV (Fig. 1A) increased from 0.91 patients per 100,000 inhabitants in 2001 to 12.84 in 2015. This increase was higher from 2001 to 2011(APC 23.62) than from 2011 to 2015 (APC 12.00). For IMV (Fig. 1B), the incidence rates decreased from 3.55 to 2.79 per 100,000 inhabitants over the entire period; however, the use of this procedure was stable from 2001 to 2009, then decreased significantly with an APC of 7.69 from 2009 to 2015. The incidence of patients who received NIV+IMV rose from 0.23 to 1.19 per 100.000 inhabitants from 2001 to 2015 (Fig. 1C). This increase was significantly from 2001 to 2009 (APC 18.93) and remained stable after that year.
Joinpoint trend analysis in the incidence of ventilatory support in hospitalized patients with community-acquired pneumonia in Spain from 2001 to 2015 according to type of ventilation. (Spansih National Hospital Discharge Database). (A) Jointpoint trend analysis in the incidence of non-invasive ventilation in hospitalized patients with community-acquired pneumonia in Spain from 2001 to 2015. (B) Jointpoint trend analysis in the incidence of invasive ventilation in hospitalized patients with community-acquired pneumonia in Spain from 2001 to 2015. (C) Jointpoint trend analysis in the incidence of non-invasive ventilation and invasive ventilation in hospitalized patients with community-acquired pneumonia in Spain from 2001 to 2015.
Over the entire study period, the use of NIV was more frequent among men than women (62.57% vs. 37.43%; p<0.001); however, the proportion of women rose significantly from 30.76% to 39.76% from the first to the last period analyzed (p<0.001) (Table 2).
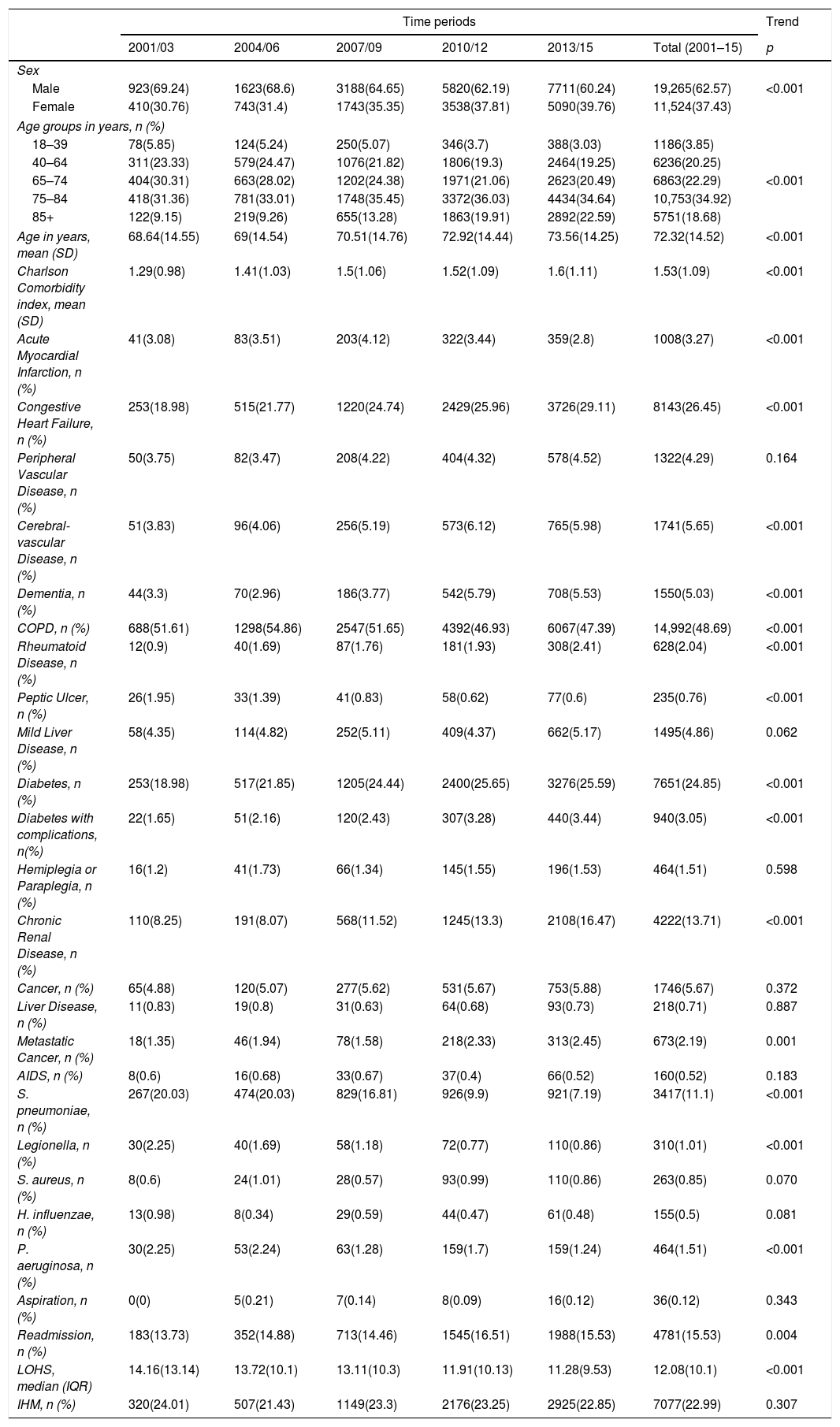
Trends in the characteristics of hospital admission with community acquired pneumonia that required non-invasive mechanical ventilation in Spain from 2001 to 2015 (Spanish National Hospital Discharge Database).
| Time periods | Trend | ||||||
|---|---|---|---|---|---|---|---|
| 2001/03 | 2004/06 | 2007/09 | 2010/12 | 2013/15 | Total (2001–15) | p | |
| Sex | |||||||
| Male | 923(69.24) | 1623(68.6) | 3188(64.65) | 5820(62.19) | 7711(60.24) | 19,265(62.57) | <0.001 |
| Female | 410(30.76) | 743(31.4) | 1743(35.35) | 3538(37.81) | 5090(39.76) | 11,524(37.43) | |
| Age groups in years, n (%) | |||||||
| 18–39 | 78(5.85) | 124(5.24) | 250(5.07) | 346(3.7) | 388(3.03) | 1186(3.85) | |
| 40–64 | 311(23.33) | 579(24.47) | 1076(21.82) | 1806(19.3) | 2464(19.25) | 6236(20.25) | |
| 65–74 | 404(30.31) | 663(28.02) | 1202(24.38) | 1971(21.06) | 2623(20.49) | 6863(22.29) | <0.001 |
| 75–84 | 418(31.36) | 781(33.01) | 1748(35.45) | 3372(36.03) | 4434(34.64) | 10,753(34.92) | |
| 85+ | 122(9.15) | 219(9.26) | 655(13.28) | 1863(19.91) | 2892(22.59) | 5751(18.68) | |
| Age in years, mean (SD) | 68.64(14.55) | 69(14.54) | 70.51(14.76) | 72.92(14.44) | 73.56(14.25) | 72.32(14.52) | <0.001 |
| Charlson Comorbidity index, mean (SD) | 1.29(0.98) | 1.41(1.03) | 1.5(1.06) | 1.52(1.09) | 1.6(1.11) | 1.53(1.09) | <0.001 |
| Acute Myocardial Infarction, n (%) | 41(3.08) | 83(3.51) | 203(4.12) | 322(3.44) | 359(2.8) | 1008(3.27) | <0.001 |
| Congestive Heart Failure, n (%) | 253(18.98) | 515(21.77) | 1220(24.74) | 2429(25.96) | 3726(29.11) | 8143(26.45) | <0.001 |
| Peripheral Vascular Disease, n (%) | 50(3.75) | 82(3.47) | 208(4.22) | 404(4.32) | 578(4.52) | 1322(4.29) | 0.164 |
| Cerebral-vascular Disease, n (%) | 51(3.83) | 96(4.06) | 256(5.19) | 573(6.12) | 765(5.98) | 1741(5.65) | <0.001 |
| Dementia, n (%) | 44(3.3) | 70(2.96) | 186(3.77) | 542(5.79) | 708(5.53) | 1550(5.03) | <0.001 |
| COPD, n (%) | 688(51.61) | 1298(54.86) | 2547(51.65) | 4392(46.93) | 6067(47.39) | 14,992(48.69) | <0.001 |
| Rheumatoid Disease, n (%) | 12(0.9) | 40(1.69) | 87(1.76) | 181(1.93) | 308(2.41) | 628(2.04) | <0.001 |
| Peptic Ulcer, n (%) | 26(1.95) | 33(1.39) | 41(0.83) | 58(0.62) | 77(0.6) | 235(0.76) | <0.001 |
| Mild Liver Disease, n (%) | 58(4.35) | 114(4.82) | 252(5.11) | 409(4.37) | 662(5.17) | 1495(4.86) | 0.062 |
| Diabetes, n (%) | 253(18.98) | 517(21.85) | 1205(24.44) | 2400(25.65) | 3276(25.59) | 7651(24.85) | <0.001 |
| Diabetes with complications, n(%) | 22(1.65) | 51(2.16) | 120(2.43) | 307(3.28) | 440(3.44) | 940(3.05) | <0.001 |
| Hemiplegia or Paraplegia, n (%) | 16(1.2) | 41(1.73) | 66(1.34) | 145(1.55) | 196(1.53) | 464(1.51) | 0.598 |
| Chronic Renal Disease, n (%) | 110(8.25) | 191(8.07) | 568(11.52) | 1245(13.3) | 2108(16.47) | 4222(13.71) | <0.001 |
| Cancer, n (%) | 65(4.88) | 120(5.07) | 277(5.62) | 531(5.67) | 753(5.88) | 1746(5.67) | 0.372 |
| Liver Disease, n (%) | 11(0.83) | 19(0.8) | 31(0.63) | 64(0.68) | 93(0.73) | 218(0.71) | 0.887 |
| Metastatic Cancer, n (%) | 18(1.35) | 46(1.94) | 78(1.58) | 218(2.33) | 313(2.45) | 673(2.19) | 0.001 |
| AIDS, n (%) | 8(0.6) | 16(0.68) | 33(0.67) | 37(0.4) | 66(0.52) | 160(0.52) | 0.183 |
| S. pneumoniae, n (%) | 267(20.03) | 474(20.03) | 829(16.81) | 926(9.9) | 921(7.19) | 3417(11.1) | <0.001 |
| Legionella, n (%) | 30(2.25) | 40(1.69) | 58(1.18) | 72(0.77) | 110(0.86) | 310(1.01) | <0.001 |
| S. aureus, n (%) | 8(0.6) | 24(1.01) | 28(0.57) | 93(0.99) | 110(0.86) | 263(0.85) | 0.070 |
| H. influenzae, n (%) | 13(0.98) | 8(0.34) | 29(0.59) | 44(0.47) | 61(0.48) | 155(0.5) | 0.081 |
| P. aeruginosa, n (%) | 30(2.25) | 53(2.24) | 63(1.28) | 159(1.7) | 159(1.24) | 464(1.51) | <0.001 |
| Aspiration, n (%) | 0(0) | 5(0.21) | 7(0.14) | 8(0.09) | 16(0.12) | 36(0.12) | 0.343 |
| Readmission, n (%) | 183(13.73) | 352(14.88) | 713(14.46) | 1545(16.51) | 1988(15.53) | 4781(15.53) | 0.004 |
| LOHS, median (IQR) | 14.16(13.14) | 13.72(10.1) | 13.11(10.3) | 11.91(10.13) | 11.28(9.53) | 12.08(10.1) | <0.001 |
| IHM, n (%) | 320(24.01) | 507(21.43) | 1149(23.3) | 2176(23.25) | 2925(22.85) | 7077(22.99) | 0.307 |
COPD: chronic obstructive pulmonary disease. LOHS: length of hospital stay. IHM: in-hospital mortality.
Trend. Showing p value for trend assessed using logistic regression adjusted by age and sex.
The mean age increased from 68.64 years in 2001–3 to 73.56 years in 2013–15 (p<0.001).
Among the chronic conditions included in the CCI, the most frequent in patients with CAP who received NIV was COPD, coded in 48.69% of patients, followed by congestive heart failure in 26.45%. The prevalence of all the conditions included in the CCI increased significantly over time (p<0.001), except acute myocardial infarction, COPD and peptic ulcer. Additionally, the mean CCI score rose from 1.29 to 1.6 (p<0.001).
Regarding pathogens isolated, S. pneumoniae, Legionella and P. aeruginosa decreased over time.
Readmissions increased from 13.73% in 2001–2003 to 15.53 in 2013–2015 (p=0.004); however, LOHS decreased significantly from 14.16 days to 9.53 days (p<0.001). Overall, 22.9% of patients with CAP who received NIV died during their hospitalization and this figure did not change significantly during the study period.
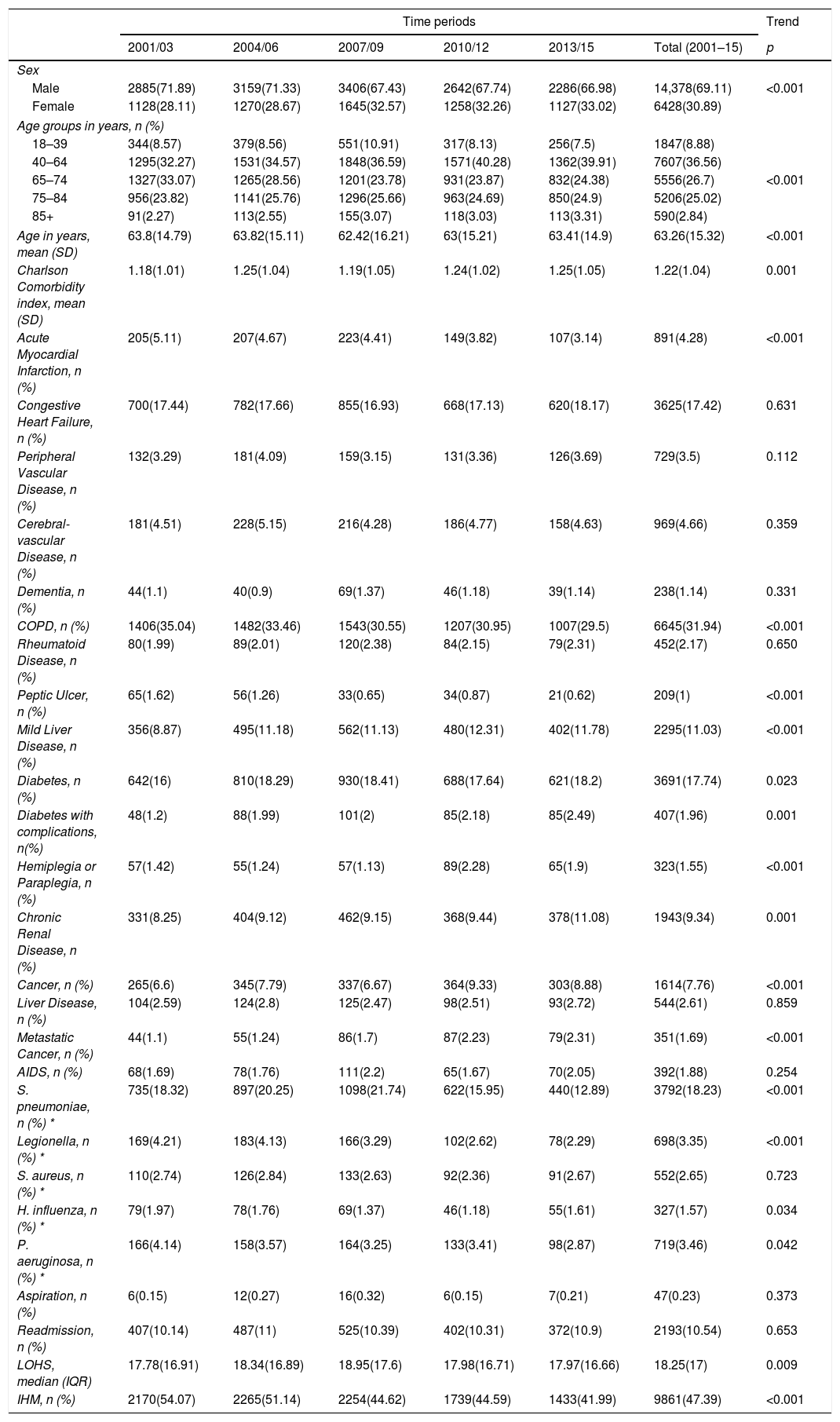
Characteristics of hospital admissions that required IMVAs seen in Table 3, from 2001 to 2015, IMV was used in a higher proportion of men than women (69.11% vs. 30.89%), however, the proportion of women rose significantly from 28.11% in 2001–2003 to 33.02% in 2013–2015. The mean age of hospitalized patients receiving this procedure decreased significantly from 63.8 years to 63.41 years from the first to the last period analyzed (both p<0.001).
Trends in the characteristics of hospital admission with community acquired pneumonia that required invasive mechanical ventilation in Spain from 2001 to 2015 (Spanish National Hospital Discharge Database).
| Time periods | Trend | ||||||
|---|---|---|---|---|---|---|---|
| 2001/03 | 2004/06 | 2007/09 | 2010/12 | 2013/15 | Total (2001–15) | p | |
| Sex | |||||||
| Male | 2885(71.89) | 3159(71.33) | 3406(67.43) | 2642(67.74) | 2286(66.98) | 14,378(69.11) | <0.001 |
| Female | 1128(28.11) | 1270(28.67) | 1645(32.57) | 1258(32.26) | 1127(33.02) | 6428(30.89) | |
| Age groups in years, n (%) | |||||||
| 18–39 | 344(8.57) | 379(8.56) | 551(10.91) | 317(8.13) | 256(7.5) | 1847(8.88) | |
| 40–64 | 1295(32.27) | 1531(34.57) | 1848(36.59) | 1571(40.28) | 1362(39.91) | 7607(36.56) | |
| 65–74 | 1327(33.07) | 1265(28.56) | 1201(23.78) | 931(23.87) | 832(24.38) | 5556(26.7) | <0.001 |
| 75–84 | 956(23.82) | 1141(25.76) | 1296(25.66) | 963(24.69) | 850(24.9) | 5206(25.02) | |
| 85+ | 91(2.27) | 113(2.55) | 155(3.07) | 118(3.03) | 113(3.31) | 590(2.84) | |
| Age in years, mean (SD) | 63.8(14.79) | 63.82(15.11) | 62.42(16.21) | 63(15.21) | 63.41(14.9) | 63.26(15.32) | <0.001 |
| Charlson Comorbidity index, mean (SD) | 1.18(1.01) | 1.25(1.04) | 1.19(1.05) | 1.24(1.02) | 1.25(1.05) | 1.22(1.04) | 0.001 |
| Acute Myocardial Infarction, n (%) | 205(5.11) | 207(4.67) | 223(4.41) | 149(3.82) | 107(3.14) | 891(4.28) | <0.001 |
| Congestive Heart Failure, n (%) | 700(17.44) | 782(17.66) | 855(16.93) | 668(17.13) | 620(18.17) | 3625(17.42) | 0.631 |
| Peripheral Vascular Disease, n (%) | 132(3.29) | 181(4.09) | 159(3.15) | 131(3.36) | 126(3.69) | 729(3.5) | 0.112 |
| Cerebral-vascular Disease, n (%) | 181(4.51) | 228(5.15) | 216(4.28) | 186(4.77) | 158(4.63) | 969(4.66) | 0.359 |
| Dementia, n (%) | 44(1.1) | 40(0.9) | 69(1.37) | 46(1.18) | 39(1.14) | 238(1.14) | 0.331 |
| COPD, n (%) | 1406(35.04) | 1482(33.46) | 1543(30.55) | 1207(30.95) | 1007(29.5) | 6645(31.94) | <0.001 |
| Rheumatoid Disease, n (%) | 80(1.99) | 89(2.01) | 120(2.38) | 84(2.15) | 79(2.31) | 452(2.17) | 0.650 |
| Peptic Ulcer, n (%) | 65(1.62) | 56(1.26) | 33(0.65) | 34(0.87) | 21(0.62) | 209(1) | <0.001 |
| Mild Liver Disease, n (%) | 356(8.87) | 495(11.18) | 562(11.13) | 480(12.31) | 402(11.78) | 2295(11.03) | <0.001 |
| Diabetes, n (%) | 642(16) | 810(18.29) | 930(18.41) | 688(17.64) | 621(18.2) | 3691(17.74) | 0.023 |
| Diabetes with complications, n(%) | 48(1.2) | 88(1.99) | 101(2) | 85(2.18) | 85(2.49) | 407(1.96) | 0.001 |
| Hemiplegia or Paraplegia, n (%) | 57(1.42) | 55(1.24) | 57(1.13) | 89(2.28) | 65(1.9) | 323(1.55) | <0.001 |
| Chronic Renal Disease, n (%) | 331(8.25) | 404(9.12) | 462(9.15) | 368(9.44) | 378(11.08) | 1943(9.34) | 0.001 |
| Cancer, n (%) | 265(6.6) | 345(7.79) | 337(6.67) | 364(9.33) | 303(8.88) | 1614(7.76) | <0.001 |
| Liver Disease, n (%) | 104(2.59) | 124(2.8) | 125(2.47) | 98(2.51) | 93(2.72) | 544(2.61) | 0.859 |
| Metastatic Cancer, n (%) | 44(1.1) | 55(1.24) | 86(1.7) | 87(2.23) | 79(2.31) | 351(1.69) | <0.001 |
| AIDS, n (%) | 68(1.69) | 78(1.76) | 111(2.2) | 65(1.67) | 70(2.05) | 392(1.88) | 0.254 |
| S. pneumoniae, n (%) * | 735(18.32) | 897(20.25) | 1098(21.74) | 622(15.95) | 440(12.89) | 3792(18.23) | <0.001 |
| Legionella, n (%) * | 169(4.21) | 183(4.13) | 166(3.29) | 102(2.62) | 78(2.29) | 698(3.35) | <0.001 |
| S. aureus, n (%) * | 110(2.74) | 126(2.84) | 133(2.63) | 92(2.36) | 91(2.67) | 552(2.65) | 0.723 |
| H. influenza, n (%) * | 79(1.97) | 78(1.76) | 69(1.37) | 46(1.18) | 55(1.61) | 327(1.57) | 0.034 |
| P. aeruginosa, n (%) * | 166(4.14) | 158(3.57) | 164(3.25) | 133(3.41) | 98(2.87) | 719(3.46) | 0.042 |
| Aspiration, n (%) | 6(0.15) | 12(0.27) | 16(0.32) | 6(0.15) | 7(0.21) | 47(0.23) | 0.373 |
| Readmission, n (%) | 407(10.14) | 487(11) | 525(10.39) | 402(10.31) | 372(10.9) | 2193(10.54) | 0.653 |
| LOHS, median (IQR) | 17.78(16.91) | 18.34(16.89) | 18.95(17.6) | 17.98(16.71) | 17.97(16.66) | 18.25(17) | 0.009 |
| IHM, n (%) | 2170(54.07) | 2265(51.14) | 2254(44.62) | 1739(44.59) | 1433(41.99) | 9861(47.39) | <0.001 |
COPD: chronic obstructive pulmonary disease. LOHS: length of hospital stay. IHM: in-hospital mortality.
Trend. Showing p value for trend assessed using logistic regression adjusted by age and sex.
The mean CCI score was 1.18 in 2001–2003, increasing to 1.25 in the last period (p=0.001). Among the clinical diseases analyzed, COPD was the most frequent in patients who received IMV, being coded in 31.94%, followed by diabetes and congestive heart failure, in 17.74% and 17.42% of patients, respectively.
In patients with CAP underwent IMV the most commonly identified pathogen was S. pneumoniae (18.23%). Of the pathogens analyzed S. pneumoniae, Legionella, H. influenzae and P. aeruginosa decreased over time (Table 3).
Over the study period, aspiration pneumonia was stable representing around 0.20%.
LOHS slightly rose from 17.78 days to 17.97 days (p=0.009). The IHM decreased significantly from 54.07% to 41.99% in patients with IMV from 2001–2003 to 2013–2015, respectively (p<0.001).
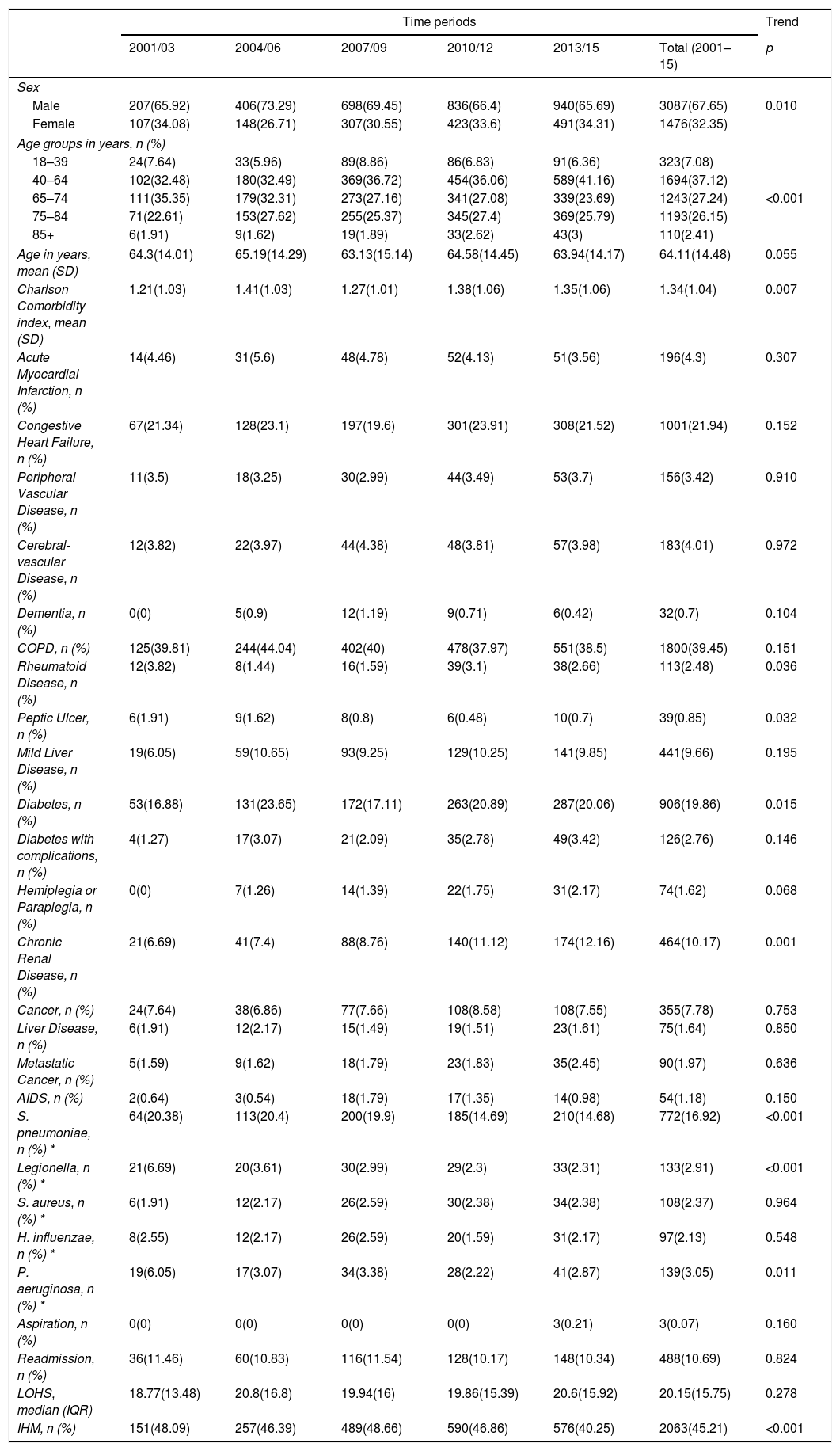
Characteristics of hospital admissions that required NIV+IMVOver the entire period, the mean age was 64.11 years and men represent 67.65% of this population with decreased from 65.92% in 2001–2003 to 65.69% in 2013–2015 (p=0.010) (Table 4).
Trends in the characteristics of hospital admission with community acquired pneumonia that required non-invasive mechanical ventilation and invasive mechanical ventilation in Spain from 2001 to 2015 (Spanish National Hospital Discharge Database).
| Time periods | Trend | ||||||
|---|---|---|---|---|---|---|---|
| 2001/03 | 2004/06 | 2007/09 | 2010/12 | 2013/15 | Total (2001–15) | p | |
| Sex | |||||||
| Male | 207(65.92) | 406(73.29) | 698(69.45) | 836(66.4) | 940(65.69) | 3087(67.65) | 0.010 |
| Female | 107(34.08) | 148(26.71) | 307(30.55) | 423(33.6) | 491(34.31) | 1476(32.35) | |
| Age groups in years, n (%) | |||||||
| 18–39 | 24(7.64) | 33(5.96) | 89(8.86) | 86(6.83) | 91(6.36) | 323(7.08) | |
| 40–64 | 102(32.48) | 180(32.49) | 369(36.72) | 454(36.06) | 589(41.16) | 1694(37.12) | |
| 65–74 | 111(35.35) | 179(32.31) | 273(27.16) | 341(27.08) | 339(23.69) | 1243(27.24) | <0.001 |
| 75–84 | 71(22.61) | 153(27.62) | 255(25.37) | 345(27.4) | 369(25.79) | 1193(26.15) | |
| 85+ | 6(1.91) | 9(1.62) | 19(1.89) | 33(2.62) | 43(3) | 110(2.41) | |
| Age in years, mean (SD) | 64.3(14.01) | 65.19(14.29) | 63.13(15.14) | 64.58(14.45) | 63.94(14.17) | 64.11(14.48) | 0.055 |
| Charlson Comorbidity index, mean (SD) | 1.21(1.03) | 1.41(1.03) | 1.27(1.01) | 1.38(1.06) | 1.35(1.06) | 1.34(1.04) | 0.007 |
| Acute Myocardial Infarction, n (%) | 14(4.46) | 31(5.6) | 48(4.78) | 52(4.13) | 51(3.56) | 196(4.3) | 0.307 |
| Congestive Heart Failure, n (%) | 67(21.34) | 128(23.1) | 197(19.6) | 301(23.91) | 308(21.52) | 1001(21.94) | 0.152 |
| Peripheral Vascular Disease, n (%) | 11(3.5) | 18(3.25) | 30(2.99) | 44(3.49) | 53(3.7) | 156(3.42) | 0.910 |
| Cerebral-vascular Disease, n (%) | 12(3.82) | 22(3.97) | 44(4.38) | 48(3.81) | 57(3.98) | 183(4.01) | 0.972 |
| Dementia, n (%) | 0(0) | 5(0.9) | 12(1.19) | 9(0.71) | 6(0.42) | 32(0.7) | 0.104 |
| COPD, n (%) | 125(39.81) | 244(44.04) | 402(40) | 478(37.97) | 551(38.5) | 1800(39.45) | 0.151 |
| Rheumatoid Disease, n (%) | 12(3.82) | 8(1.44) | 16(1.59) | 39(3.1) | 38(2.66) | 113(2.48) | 0.036 |
| Peptic Ulcer, n (%) | 6(1.91) | 9(1.62) | 8(0.8) | 6(0.48) | 10(0.7) | 39(0.85) | 0.032 |
| Mild Liver Disease, n (%) | 19(6.05) | 59(10.65) | 93(9.25) | 129(10.25) | 141(9.85) | 441(9.66) | 0.195 |
| Diabetes, n (%) | 53(16.88) | 131(23.65) | 172(17.11) | 263(20.89) | 287(20.06) | 906(19.86) | 0.015 |
| Diabetes with complications, n (%) | 4(1.27) | 17(3.07) | 21(2.09) | 35(2.78) | 49(3.42) | 126(2.76) | 0.146 |
| Hemiplegia or Paraplegia, n (%) | 0(0) | 7(1.26) | 14(1.39) | 22(1.75) | 31(2.17) | 74(1.62) | 0.068 |
| Chronic Renal Disease, n (%) | 21(6.69) | 41(7.4) | 88(8.76) | 140(11.12) | 174(12.16) | 464(10.17) | 0.001 |
| Cancer, n (%) | 24(7.64) | 38(6.86) | 77(7.66) | 108(8.58) | 108(7.55) | 355(7.78) | 0.753 |
| Liver Disease, n (%) | 6(1.91) | 12(2.17) | 15(1.49) | 19(1.51) | 23(1.61) | 75(1.64) | 0.850 |
| Metastatic Cancer, n (%) | 5(1.59) | 9(1.62) | 18(1.79) | 23(1.83) | 35(2.45) | 90(1.97) | 0.636 |
| AIDS, n (%) | 2(0.64) | 3(0.54) | 18(1.79) | 17(1.35) | 14(0.98) | 54(1.18) | 0.150 |
| S. pneumoniae, n (%) * | 64(20.38) | 113(20.4) | 200(19.9) | 185(14.69) | 210(14.68) | 772(16.92) | <0.001 |
| Legionella, n (%) * | 21(6.69) | 20(3.61) | 30(2.99) | 29(2.3) | 33(2.31) | 133(2.91) | <0.001 |
| S. aureus, n (%) * | 6(1.91) | 12(2.17) | 26(2.59) | 30(2.38) | 34(2.38) | 108(2.37) | 0.964 |
| H. influenzae, n (%) * | 8(2.55) | 12(2.17) | 26(2.59) | 20(1.59) | 31(2.17) | 97(2.13) | 0.548 |
| P. aeruginosa, n (%) * | 19(6.05) | 17(3.07) | 34(3.38) | 28(2.22) | 41(2.87) | 139(3.05) | 0.011 |
| Aspiration, n (%) | 0(0) | 0(0) | 0(0) | 0(0) | 3(0.21) | 3(0.07) | 0.160 |
| Readmission, n (%) | 36(11.46) | 60(10.83) | 116(11.54) | 128(10.17) | 148(10.34) | 488(10.69) | 0.824 |
| LOHS, median (IQR) | 18.77(13.48) | 20.8(16.8) | 19.94(16) | 19.86(15.39) | 20.6(15.92) | 20.15(15.75) | 0.278 |
| IHM, n (%) | 151(48.09) | 257(46.39) | 489(48.66) | 590(46.86) | 576(40.25) | 2063(45.21) | <0.001 |
COPD: chronic obstructive pulmonary disease. LOHS: length of hospital stay. IHM: in-hospital mortality.
Trend. Showing p value for trend assessed using logistic regression adjusted by age and sex.
As described in patients who received isolated NIV and IMV, the mean CCI score increased from 1.21 to 1.35 over the study period (p=0.007). Among the clinical conditions analyzed, the most frequent in patients who received NIV+IMV was COPD (39.45%), followed by congestive heart failure (21.94%) and diabetes (19.86%).
From 2001–2003 to 2013–2015, S. pneumoniae, Legionella and H. influenzae decreased significantly from 20.38% and 6.69% to 14.68% and 2.31%, respectively in 2001–2003 to 14.68%, 2.31% and 2.87% in 2013–2015.
Readmission and the mean LOHS remained stable at approximately 10% and 20 days, respectively; whereas IHM decreased significantly from 2001–2003 (48.09%) to 2013–2015 (40.25%).
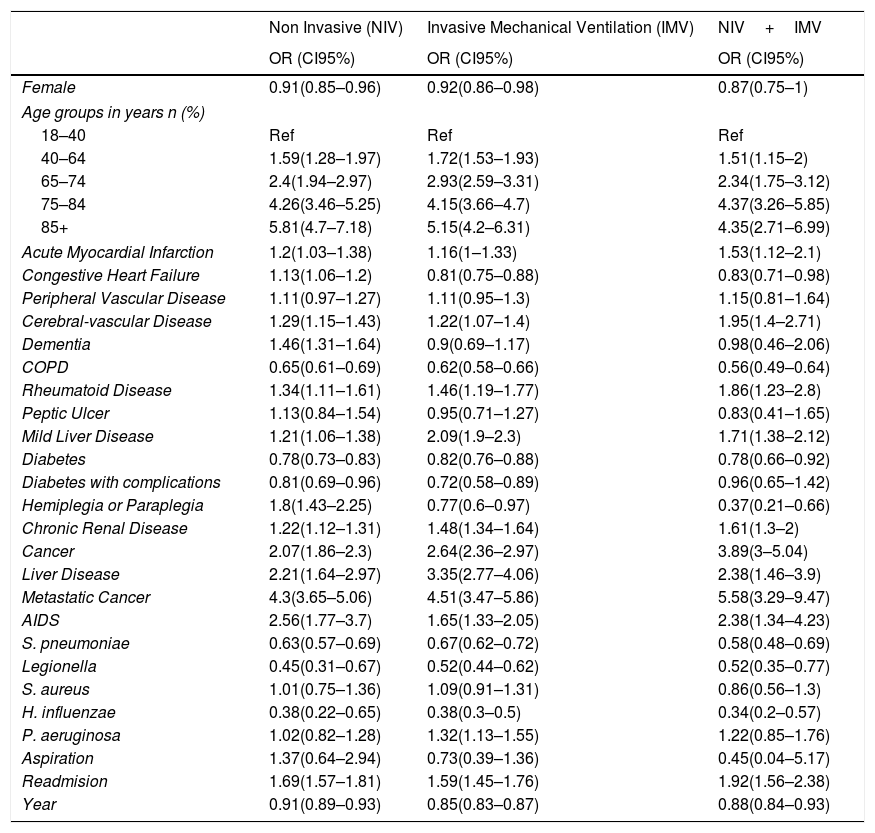
Time trend and factors associated with in-hospital mortality in patients with CAPFemale sex had a protective effect for IHM among those with requiring NIV and IMV.
Older age was a significant risk factor for IHM in the three groups analyzed (Table 5).
Multivariable logistic regression models to assess time trend and to identify factors associated with IHM for each ventilator support type.
| Non Invasive (NIV) | Invasive Mechanical Ventilation (IMV) | NIV+IMV | |
|---|---|---|---|
| OR (CI95%) | OR (CI95%) | OR (CI95%) | |
| Female | 0.91(0.85–0.96) | 0.92(0.86–0.98) | 0.87(0.75–1) |
| Age groups in years n (%) | |||
| 18–40 | Ref | Ref | Ref |
| 40–64 | 1.59(1.28–1.97) | 1.72(1.53–1.93) | 1.51(1.15–2) |
| 65–74 | 2.4(1.94–2.97) | 2.93(2.59–3.31) | 2.34(1.75–3.12) |
| 75–84 | 4.26(3.46–5.25) | 4.15(3.66–4.7) | 4.37(3.26–5.85) |
| 85+ | 5.81(4.7–7.18) | 5.15(4.2–6.31) | 4.35(2.71–6.99) |
| Acute Myocardial Infarction | 1.2(1.03–1.38) | 1.16(1–1.33) | 1.53(1.12–2.1) |
| Congestive Heart Failure | 1.13(1.06–1.2) | 0.81(0.75–0.88) | 0.83(0.71–0.98) |
| Peripheral Vascular Disease | 1.11(0.97–1.27) | 1.11(0.95–1.3) | 1.15(0.81–1.64) |
| Cerebral-vascular Disease | 1.29(1.15–1.43) | 1.22(1.07–1.4) | 1.95(1.4–2.71) |
| Dementia | 1.46(1.31–1.64) | 0.9(0.69–1.17) | 0.98(0.46–2.06) |
| COPD | 0.65(0.61–0.69) | 0.62(0.58–0.66) | 0.56(0.49–0.64) |
| Rheumatoid Disease | 1.34(1.11–1.61) | 1.46(1.19–1.77) | 1.86(1.23–2.8) |
| Peptic Ulcer | 1.13(0.84–1.54) | 0.95(0.71–1.27) | 0.83(0.41–1.65) |
| Mild Liver Disease | 1.21(1.06–1.38) | 2.09(1.9–2.3) | 1.71(1.38–2.12) |
| Diabetes | 0.78(0.73–0.83) | 0.82(0.76–0.88) | 0.78(0.66–0.92) |
| Diabetes with complications | 0.81(0.69–0.96) | 0.72(0.58–0.89) | 0.96(0.65–1.42) |
| Hemiplegia or Paraplegia | 1.8(1.43–2.25) | 0.77(0.6–0.97) | 0.37(0.21–0.66) |
| Chronic Renal Disease | 1.22(1.12–1.31) | 1.48(1.34–1.64) | 1.61(1.3–2) |
| Cancer | 2.07(1.86–2.3) | 2.64(2.36–2.97) | 3.89(3–5.04) |
| Liver Disease | 2.21(1.64–2.97) | 3.35(2.77–4.06) | 2.38(1.46–3.9) |
| Metastatic Cancer | 4.3(3.65–5.06) | 4.51(3.47–5.86) | 5.58(3.29–9.47) |
| AIDS | 2.56(1.77–3.7) | 1.65(1.33–2.05) | 2.38(1.34–4.23) |
| S. pneumoniae | 0.63(0.57–0.69) | 0.67(0.62–0.72) | 0.58(0.48–0.69) |
| Legionella | 0.45(0.31–0.67) | 0.52(0.44–0.62) | 0.52(0.35–0.77) |
| S. aureus | 1.01(0.75–1.36) | 1.09(0.91–1.31) | 0.86(0.56–1.3) |
| H. influenzae | 0.38(0.22–0.65) | 0.38(0.3–0.5) | 0.34(0.2–0.57) |
| P. aeruginosa | 1.02(0.82–1.28) | 1.32(1.13–1.55) | 1.22(0.85–1.76) |
| Aspiration | 1.37(0.64–2.94) | 0.73(0.39–1.36) | 0.45(0.04–5.17) |
| Readmision | 1.69(1.57–1.81) | 1.59(1.45–1.76) | 1.92(1.56–2.38) |
| Year | 0.91(0.89–0.93) | 0.85(0.83–0.87) | 0.88(0.84–0.93) |
COPD: chronic obstructive pulmonary disease.
Basically, all the conditions included in the CCI increased the risk for IHM in patients with CAP and with ventilator support except for COPD and diabetes. These two conditions reduce the risk for IHM in NIV, IMV and NIV+IMV. Congestive heart failure and hemiplegia/paraplegia reduce the risk for IHM in IMV and NIV+IMV.
Presence of S. pneumoniae, Legionella and H. influenzae reduce the risk for IHM in patients with CAP and with ventilator support. However, P. aeruginosa increased the risk of dying in patients with IMV (OR 1.32; 95%CI 1.13–1.55).
Being a readmission increased the probability of IHM in all the types of ventilator support analyzed.
Finally, after adjusting for possible confounders, IHM decreased significantly from 2001 to 2015 in Spain in patients with CAP who received NIV, IMV and NIV+IMV.
DiscussionOur current study shows that most patients hospitalized with CAP who required ventilation received NIV as the only ventilation method. In addition, the use of NIV increased over time, as well as the incidence of patients who received NIV+IMV. Over the last decade, NIV use has significantly increased in patients with pneumonia,22 despite the fact that only few randomized controlled trials have been published to date assessing the effectiveness of this procedure.2,23–25. In fact, the ERS/ATS guidelines on the use of NIV in acute respiratory failure recognize that evidence in this setting is insufficient to recommend its routine use, in view of the specific risks associated with the use of this therapeutic modality.26 However, taking into account that some studies have identified populations in whom the chances of success are higher, it has been suggested that NIV can be attempted in patients with CAP if the following conditions are met: hypoxemic respiratory failure, management by an experienced clinical team, meticulous patient selection (careful exclusion of contraindications, such as altered mental status, shock, or multiorgan failure), close monitoring in an intensive care unit, and early reevaluation after starting NIV, with a prompt switch to intubation if no improvement is observed. The objectives of NIV in these circumstances are to improve oxygenation, facilitate ventilation, reduce the work of breathing and dyspnea, avoid intubation, and prevent the complications associated with the use of IMV.27
In patients treated with NIV, we found an increase of CCI score over time. Despite it, we cannot establish how much of this increase it was due to better coding and how much to the increased complexity of the casemix. Nevertheless, the SNHDD receives periodical audits to warrantee its validity, which support the second option.28 Among the chronic conditions included in the CCI, the most frequently found in these patients was COPD, followed by congestive heart failure. One explication for the higher use of NIV than IMV in patients with comorbid COPD and/or heart failure could be that physicians are likely to consider using NIV in these populations for which NIV efficacy is well established. Other possibility is that the increased levels of ventilation perfusion mismatch and higher minute ventilation in the context of pneumonia are poorly tolerated in these patients and as a consequence, this group exhibits signs of respiratory failure earlier that those without COPD and/or heart failure.29
The marked increase in the use of NIV in our study paired with a reduction in the use of IMV from 20012 to 2015. This finding adds to evidence from other studies which have similarly reported a dramatic increase in the use of NIV and a decrease in the use of IMV in patients with acute respiratory failure of different etiologies.30 Conversely, Mehta et al. evidenced that IMV use increased in US from 1993–2009 and found that pneumonia accounted for a large portion of this increase.18 They suggested that increasing number of comorbidities as well as an aging US population may have increased the number of individuals with pneumonia, resulting in higher numbers requiring IMV. However, we also found an increase in the mean age at admission and in the mean CCI score during the study period and, despite this, we observed a decrease in the use of IMV over time.
Regarding pathogens isolated, S. pneumoniae decreased over time in patients with CAP underwent NIV or IMV. In this way, Yin et al. have also found that the proportion of CAP attributable to S. pneumoniae has been declining in Australian adults.31 In this way, Vestjens et al. have observed that the proportion of pneumococcal CAP have decreased over time in adults in The Netherlands after introduction of pneumococcal conjugate vaccine in infants.32
When we made comparisons between hospital admissions according to ventilator support type, we found that patients receiving NIV were the oldest and those who underwent IMV were the youngest. On the other hand, patients who required NIV had the highest mean CCI score, with IMV having the lowest values. The highest mean LOHS was found in those who received NIV+IMV and the lowest, in those who received NIV. However, NIV group had the highest readmission rate. Finally, patients who received only IMV had the highest IHM, followed by those with NIV+IMV and only NIV. In a previous study, Stefan et al. also compared the outcomes of patients hospitalized with pneumonia treated with NIV and IMV.5 As in our case, they found that patients initially treated with NIV were older than those initially with IMV. Comorbidities such as COPD and congestive heart failure also were more frequent among those treated with NIV in their study. In addition, patients treated with NIV had shorter LOHS but there were no significant differences in 30-day readmission rate. Furthermore, NIV therapy was associated with a 29% relative reduction of IHM compared with IMV. It can be justified, at least in part, by the fact that the sickest patients receiving invasive mechanical ventilation. In a more recent study, Valley et al. found that among marginal patients with pneumonia there were no difference in mortality between both types of ventilation.33 However, their analysis was restricted to the elderly, they exclude patients with comorbid COPD and cardiogenic pulmonary edema, their primary outcome was 30-day all-cause mortality not IHM and their results applied to the marginal patients not to the average ventilated patients as in our case.33
We found that factors associated with IHM were different for each ventilator support type. Older age was a significant risk factor for IHM in the three groups analyzed. Most of the conditions included in the CCI increased the risk for IHM in patients with CAP who received ventilatory support. Burden of comorbidities has been previously identified as a factor independently associated with IHM.34 However, COPD reduced the risk for IHM in NIV, IMV and NIV+IMV and congestive heart failure reduced the risk for IHM in IMV and NIV+IMV in our study. In this regard, Stefan et al. found that initial NIV was associated with better survival compared to initial IMV in patients hospitalized with pneumonia, but only among those with these comorbid cardiopulmonary conditions.5 It is possible that acute respiratory failure in patients with pneumonia superimposed on COPD and/or heart failure may be more evident earlier in the presence of these comorbidities, which respond better to NIV.2,35,36 Unfortunately given the characteristic of the SNHDD is not possible to determine the precise cause of acute respiratory failure for each patient. On the other hand, readmission increased the probability of IHM in all the types of ventilator support analyzed in the present study. Lastly, after adjusting for possible confounders, IHM decreased significantly from 2001 to 2015 in Spain in patients with CAP who received NIV, IMV and NIV+IMV. Vallés et al. also found a decrease in ICU mortality in Spain when they studied the characteristics and outcomes of patients with severe CAP over a 15-year surveillance period (1999–2013), despite a progressively higher incidence and severity of this disease in their ICU.37 In any case, it is possible that changes in hospital protocols, national guidelines or ventilatory strategies over time may have contributed to a reduction in the IHM. In this way, Costantini et al. have found that compliance with guidelines change over time, with some effects on mortality and with an apparent reduction in the duration of antibiotic therapy and in the length of hospital stay.38 More recently, Simonetti et al. have reported that 30-day mortality decrease significantly over time in hospitalized patients with CAP in spite of an upward trend in patient age and other factors associated with poor outcomes, and they have suggested that several changes in the management of CAP and a general improvement in global care over time may justified these results.39
Our study has several limitations. We lacked data on physiologic parameters, non-ventilatory medical treatment, and other factors that may affect mortality, such as a “do not intubate” order. Thereby, patients with acute respiratory failure and an order not to intubate could have been offered NIV as a “ceiling treatment”. Nevertheless, this bias would increase mortality in the NIV group. Regarding the use of NIV+IMV, we cannot establish which of the two types of ventilatory support was used in the first place and the sequence could affect the hospital outcomes. However, this group is very small compared to patients who received NIV or IMV in isolation so in our opinion the effect, if any, on the conclusions of our investigations would be not relevant.
ConclusionsThe current study allows clarifying the knowledge related to epidemiological trends of the use of mechanical ventilation, both invasive and non-invasive, in patients with CAP in real life setting. We demonstrated larges increases in NIV use and significant decreases in IMV utilization, as well as significant changes in hospital outcomes over time, which may have implications in the future allocation of health resources.
Conflict of interestThe authors declare no conflict of interest..