Sarcoidosis is a multifaceted systemic inflammatory disorder that can involve virtually any organ system in the body. This condition is characterized by the formation of granulomas, which are small clusters of immune cells that can lead to various symptoms and complications that ultimately impact organ function.1 Presently, there is no specific genetic biomarker to facilitate the diagnosis or predict the prognosis of this complex illness.
In recent decades, there has been a substantial increase in genetic studies investigating disease associations, and this trend is anticipated to continue over the coming years as research efforts expand through the utilization of biobanks worldwide. The initial genome-wide association study (GWAS) that paved the way for subsequent investigations was conducted by the Wellcome Trust Case Control Consortium (WTCC) in 2007.2 GWAS studies have revealed millions of associations related to human traits and diseases.3
One of the primary factors contributing to the efficacy of GWAS across various fields—such as cardiovascular diseases, metabolic disorders, and autoimmune conditions—has been twofold. Firstly, researchers have refined their methodologies for meticulously characterizing phenotypes and disease traits. This careful characterization is essential, as it allows scientists to dissect the complexities of these diseases at a molecular level and facilitates the identification of precise genetic signals associated with each condition. Secondly, substantial federal funding directed toward advancing research in these areas has provided the requisite resources for comprehensive research and translational studies. In stark contrast, sarcoidosis—a disease that significantly impacts health, diminishes the quality of life and may lead to long-term disability and psychological burdens4—has not received the same level of attention as other chronic diseases. Despite the considerable burden that sarcoidosis imposes on patients and the healthcare system,5 government funders and policymakers frequently neglect the societal impact of this disease.
Epidemiological studies have highlighted significant variations in the presentation of sarcoidosis, which can be influenced by factors such as race, ethnicity, sex, age, geographic location, occupation, and lifestyle behaviors. A notable research initiative in this domain was a multicenter study known as the A Case Control Etiologic Study of Sarcoidosis (ACCESS Study).6 The findings from the ACCESS study underscored notable familial aggregation in sarcoidosis, suggesting the presence of an underlying genetic component.7 This discovery has fostered increased interest in exploring the genetic factors contributing to the risk of sarcoidosis, thereby highlighting the need for further research in this critical area.
Recent epidemiological studies highlight significant health disparities faced by sarcoidosis patients, especially among Black individuals and women, who encounter challenges impacting their quality of life8–10 and lung function.11 Analyzing the relationship between genetic susceptibility and social determinants through gene-environment interactions can provide insights into why some groups are more affected. This understanding can help develop targeted interventions, promote health equity, and improve patient-reported outcomes (PROs).
The study of genetics in sarcoidosis traces back to the 1920s, with the initial identification of familial sarcoidosis. By the 1970s, research began to pivot toward examining candidate genes, particularly focusing on human leukocyte antigen (HLA) genes, especially HLA-DRB1.12–14 Following the discovery of these HLA genes, other relevant genes also came to light. A significant milestone came with the first genome-wide scan by Schürmann et al.,15 which pointed to the BTNL2 gene as a possible contributor to the development of sarcoidosis. This crucial work set the stage for a more comprehensive understanding of the genetic factors underlying this complex disease.
Almost two decades ago, a landmark GWAS utilized high-throughput genotyping methods based on single-nucleotide polymorphisms (SNPs) within a German cohort, uncovering the ANXA11 gene as a susceptibility locus—a significant achievement in this field.16 Since that initial study, the genetic research landscape has continued to progress, albeit at a more measured pace than seen with other complex human diseases. Follow-up studies on sarcoidosis have underscored the importance of HLA-DRB1, BTNL2, and ANXA11 genes, which are associated with sarcoidosis and its clinical endophenotypes across various populations. However, these genetic markers have not yet been integrated into routine clinical practices.
Sweden is notable for including HLA-DRB1 genotyping in diagnosing sarcoidosis, particularly in Löfgren's syndrome (LS). This genetic marker provides essential insights into prognosis and helps healthcare providers tailor management plans, optimize treatments, and schedule follow-ups, ultimately enhancing patient care.
As of January 23, 2025, the NHGRI–EBI GWAS Catalog contains over 7125 GWAS publications and more than 7 million significant associations (P<5e−8), serving as a vital resource for the scientific community.17 Genetic discoveries from GWAS have facilitated the development of precise diagnostic tools across various medical specialties. Notably, 13 GWAS related to sarcoidosis have been published, indicating growing research interest in its genetics.
Challenges persist in securing funding for genetic research in sarcoidosis, particularly due to the diversity of clinical phenotypes that complicates identifying genetic signals. Establishing a foundation based on common genetic variants, especially SNPs with a minor allele frequency (MAF) of at least 5%, is vital. Detailed phenotype characterization is crucial for uncovering significant genetic associations, which can enhance diagnosis, treatment, and understanding of disease heterogeneity.
The absence of diverse multi-ethnic data poses a significant challenge in understanding the genetics of sarcoidosis across different populations. By integrating information about common genetic variants and associated phenotypes, we can uncover valuable insights and pave the way for further exploration into genetic foundations, particularly rare variants and omics approaches.
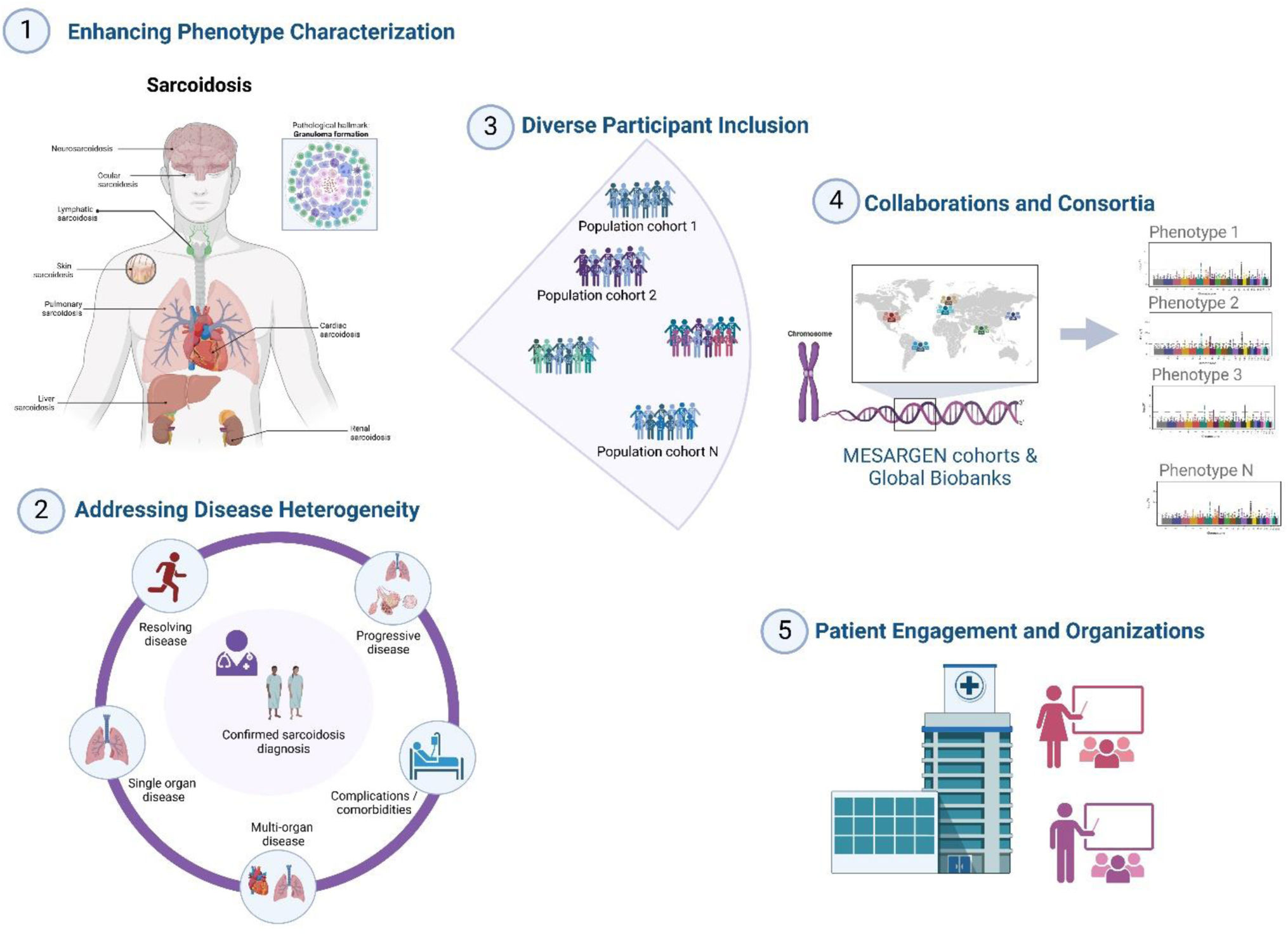
To foster meaningful advancements in genomic research related to sarcoidosis and enable impactful translational studies, it is crucial to concentrate on several key areas (illustrated in Fig. 1):
- 1.
Enhancing Phenotype Characterization: Developing an accurate phenotype characterization is essential, involving the identification and categorization of sarcoidosis clinical manifestations by severity, progression, and treatment response.
- 2.
Addressing Disease Heterogeneity: By conducting comprehensive studies that focus on the different subtypes (or endophenotypes) of sarcoidosis coupled with genotypes, researchers can better quantify and elucidate the genetic contributions to these varied manifestations.
- 3.
Diverse Participant Inclusion: The inclusion of multi-ethnic populations and different ancestries in research studies is crucial. Such population diversity will enhance the statistical power of the studies and ensure that the genetic and environmental influences on sarcoidosis are accurately represented.
- 4.
Collaborations and Consortia: Collaborating across institutions through consortia, like the Multi-Ethnic Sarcoidosis Genomics Consortium – MESARGEN and SARCOIDOMICS can significantly accelerate genetic research on sarcoidosis. By combining resources, expertise, and data, these partnerships enable large-scale studies that individual researchers or smaller groups may not achieve alone.
- 5.
Patient Engagement and Organizations: Engaging patients and collaborating with patient organizations amplifies research reach and ensures patient perspectives are included in the research process.
We can deepen our understanding of sarcoidosis and its genetic foundations by tackling these significant challenges. Ultimately, these collective efforts could pave the way for more effective interventions and tailored treatment strategies for those affected by this complex condition.
It is also crucial to consider ethical implications, ensuring compliance with regulations like GDPR, obtaining institutional review board approvals, and securing informed consent from participants. Strong data protection measures must be in place to safeguard genetic information.
Lastly, adhering to the FAIR principles in data sharing, while prioritizing participant privacy, will enhance the integrity of genomic research in sarcoidosis and drive meaningful advancements in the field.
FundingThis work was supported by the Swedish Heart-Lung Foundation (Nr. 20200505 and 20200506) to NVR.
Conflict of InterestNo conflict of interest.