Chronic thromboembolic pulmonary hypertension (CTEPH) results from obstruction of the pulmonary arterial bed by organized thrombus after acute or recurrent pulmonary embolism (PE). Its pathogenesis associates small-vessel vasculopathy. The resultant increased pulmonary pressures may lead to right ventricular dysfunction and death. CPETH patients are usually aged and present with comorbidities1 in contrast with the usual demographics in the pulmonary arterial hypertension (PAH) population. As CTEPH is consequence of thrombus formation, lifelong anticoagulation is mandatory; while treatments to address increased pulmonary pressures include: pulmonary endarterectomy (PEA), percutaneous balloon pulmonary angioplasty (BPA), and PAH-specific medication.1,2
The clinical picture of the new coronavirus disease of 2019 (COVID-19) varies greatly, ranging from asymptomatic cases to a severe acute respiratory distress syndrome (ARDS) responsible for most COVID-19 fatalities.3 A severe course occurs more likely in patients with previous cardiac or respiratory conditions. Noteworthy, coagulopathy appears to play a substantial role in COVID-19 pathogenesis.4
By May the 3rd, three CTEPH patients had been diagnosed with COVID-19 pneumonia. Baseline characteristics and COVID-19 course are presented in Table 1.
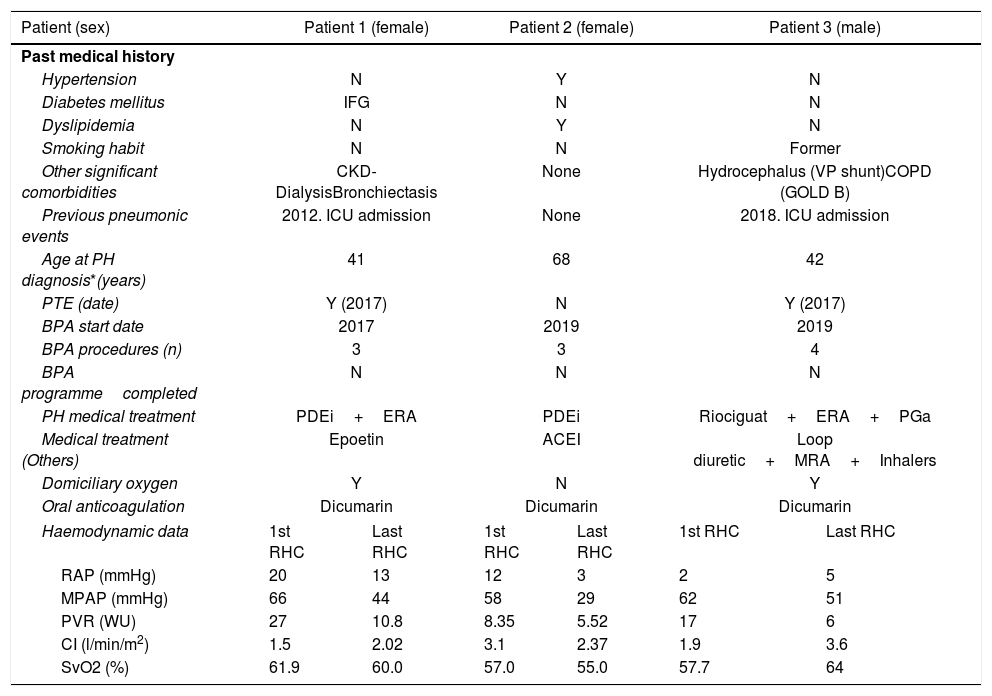
Summary of previous medical history and COVID-19 clinical picture in 3 CTEPH patients.
| Patient (sex) | Patient 1 (female) | Patient 2 (female) | Patient 3 (male) | |||
|---|---|---|---|---|---|---|
| Past medical history | ||||||
| Hypertension | N | Y | N | |||
| Diabetes mellitus | IFG | N | N | |||
| Dyslipidemia | N | Y | N | |||
| Smoking habit | N | N | Former | |||
| Other significant comorbidities | CKD-DialysisBronchiectasis | None | Hydrocephalus (VP shunt)COPD (GOLD B) | |||
| Previous pneumonic events | 2012. ICU admission | None | 2018. ICU admission | |||
| Age at PH diagnosis*(years) | 41 | 68 | 42 | |||
| PTE (date) | Y (2017) | N | Y (2017) | |||
| BPA start date | 2017 | 2019 | 2019 | |||
| BPA procedures (n) | 3 | 3 | 4 | |||
| BPA programmecompleted | N | N | N | |||
| PH medical treatment | PDEi+ERA | PDEi | Riociguat+ERA+PGa | |||
| Medical treatment (Others) | Epoetin | ACEI | Loop diuretic+MRA+Inhalers | |||
| Domiciliary oxygen | Y | N | Y | |||
| Oral anticoagulation | Dicumarin | Dicumarin | Dicumarin | |||
| Haemodynamic data | 1st RHC | Last RHC | 1st RHC | Last RHC | 1st RHC | Last RHC |
| RAP (mmHg) | 20 | 13 | 12 | 3 | 2 | 5 |
| MPAP (mmHg) | 66 | 44 | 58 | 29 | 62 | 51 |
| PVR (WU) | 27 | 10.8 | 8.35 | 5.52 | 17 | 6 |
| CI (l/min/m2) | 1.5 | 2.02 | 3.1 | 2.37 | 1.9 | 3.6 |
| SvO2 (%) | 61.9 | 60.0 | 57.0 | 55.0 | 57.7 | 64 |
| COVID-19 course | |||
|---|---|---|---|
| Age at COVID-19 (years) | 44 | 69 | 46 |
| Length of stay (days) | 10 | 8 | 13 |
| Clinical data at admission | |||
| Symptoms | ExpectorationDysthermiaMyalgiaMild dyspnoea | HeadacheDysthermiaDiarrhoeaMild dyspnoea | DysthermiaMyalgiaMild dyspnoea |
| Temperature (°C) | 38.3 | 38.0 | 38.0 |
| SpO2 (%) | 99 | 92 | 90 |
| Blood pressure (mmHg) | 70/36 | 105/70 | 110/57 |
| Chest X-ray pattern | Bilobar infiltrate | Unilobar infiltrate | Bilobar infiltrate |
| Laboratory findings | |||
| Lymphocytes (per μL) | 792 | 1400 | 600 |
| Platelet count (per μL) | 83,000 | 134,000 | 222,000 |
| Haemoglobin (g/dL) | 12.6 | 13.1 | 14.1 |
| Fibrinogen (mg/dL) | >500 | NA | 699 |
| d-Dimer (ng/mL) | NA | NA | 353 |
| Lactate dehydrogenase (U/L) | 279 | 251 | NA |
| C-Reactive Protein (mg/dL) | 9.39 | 1.35 | 28.3 |
| Treatment received | |||
| Lopinavir/Ritonavir (days) | Y (3) | N | N |
| Hydroxychloroquine (days) | Y (5) | N | Y (5) |
| Ceftriaxone (days) | Y (7) | N | N |
| Azithromycin (days) | Y (7) | N | Y (7) |
| Steroids | N | N | Y (3) |
| Anti-IL6 | N | N | Y (1) |
| Oxygen therapy (l/min) | Nasal cannula (3) | N | Nasal cannula (2) |
ACEI=Angiotensin-converting enzyme inhibitors; BPA=Balloon pulmonary angioplasty; CKD=Chronic Kidney Disease; COPD=Chronic Obstructive Pulmonary Disease; CI=Cardiac index; COVID-19=Coronavirus disease 2019; ERA=endothelin receptor antagonists; IFG=Impaired Fasting Glucose; MPAP=Mean pulmonary artery pressure; MRA=mineralocorticoids receptor antagonists; NA=not available; N=No; PDEi=phosphodiesterase inhibitors; PGa=prostaglandin analogue; PH=pulmonary hypertension; PTE=pulmonary thromboendarterectomy; PVR=Pulmonary vascular resistances; RAP=Right atrial pressure; SpO2=Peripheral oxygen saturation; SvO2=Venous oxygen saturation; VP=ventriculoperitoneal (shunt); Y=Yes.
Patient 1 was a 44-year-old female diagnosed with CTEPH at the age of 41. Other previous conditions included chronic kidney disease (CKD) on haemodialysis, bronchiectasis and a previous severe pneumonia. She underwent PEA in 2017, later enrolled in our BPA programme. She was on phosphodiesterase-5 inhibitors (PDEi), endothelin receptor antagonists (ERA) and domiciliary oxygen. In March 2020, she was admitted to hospital with poor general condition, expectoration and mild dyspnoea. Chest X-ray exhibited bilateral pneumonia. Nasal swab SARS-CoV-2 PCR was positive. She was started on antivirals, antibiotics and hydroxychloroquine. Occasional increase in oxygen flow rate was required. She was discharged home after a 10-day hospitalization period.
Patient 2 was a 70-year-old female diagnosed with CTEPH two years before, then started on sildenafil and included in our BPA programme. By mid-March 2020 she presented to hospital with high fever and general malaise. Chest X-ray showed unilobar pneumonia. SARS-CoV-2 PCR on nasal swab was positive. According to hospital protocols, she was offered treatment with ritonavir/lopinavir and azithromycin, but she declined. Still, she presented a satisfactory recovery with symptomatic treatment being discharged after 8 days of admission.
Patient 3 was a 46-year-old male with chronic obstructive pulmonary disease and CTEPH diagnosed at the age of 43. PEA was performed in 2017, and subsequently included in our BPA programme. He maintained triple vasodilator therapy and domiciliary oxygen. He also had history of critical pneumonia requiring invasive ventilation. In April 2020, he was admitted to hospital with high fever, mild dyspnoea and myalgias related to COVID-19 bilobar pneumonia (Fig. 1). CRP was remarkably high (28.3mg/dL) but SARS-CoV-2 PCR was negative, other viral panel and blood cultures were negative and there was no sign of bacterial superinfection. The epidemiological context, clinical presentation, lab results and radiographic pattern led to assume a false negative, and he was managed as COVID-19, with excessive inflammation governing the clinical picture. Accordingly, steroids and tocilizumab were prescribed along with antibiotics, with excellent response over the first two days. Hospital discharge was postponed due to readjustments on prostacyclin-administration route.
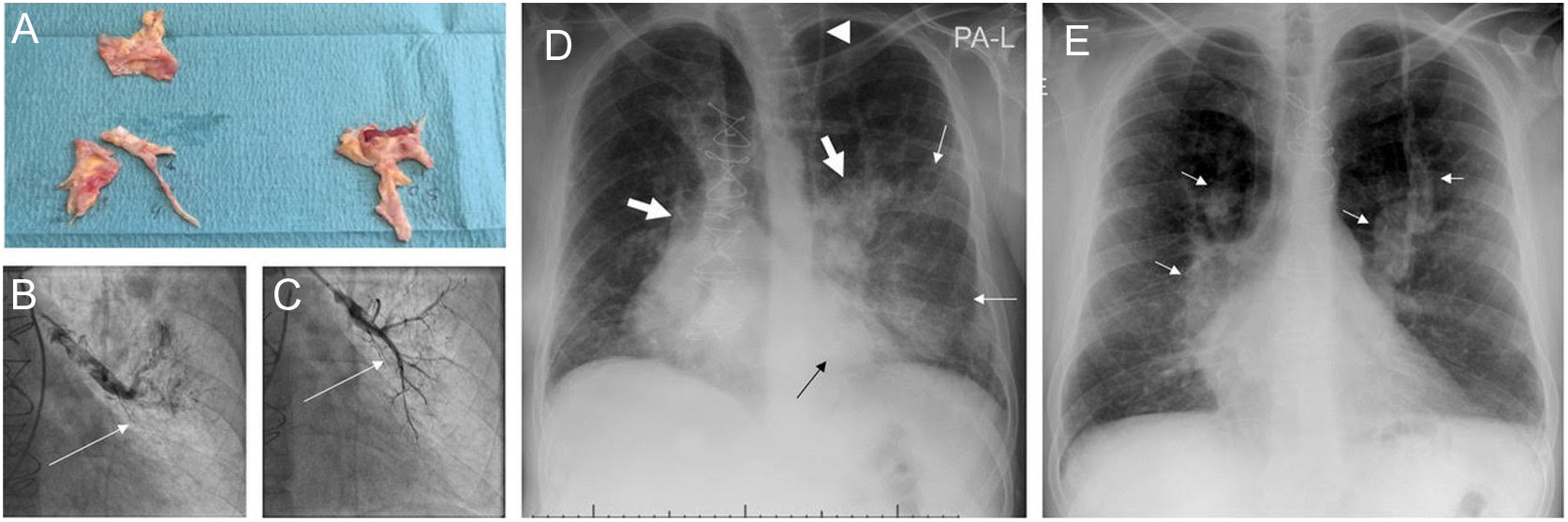
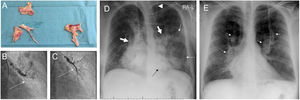
Images from patient 3. A. Material removed from the pulmonary vasculature by pulmonary endarterectomy. B. Pulmonary angiography showing occlusion (arrow) of the anterior segmental artery for the left superior lobe prior to percutaneous treatment of this region. C. The anterior segmental artery for the left superior lobe is recanalized after balloon pulmonary angioplasty (arrow). D. Chest X-ray on admission showing Ill-defined consolidations in medium and inferior left pulmonary fields (thin arrows). Note the dilation of the main pulmonary arteries (thick arrows) and the sternotomy wires. Interestingly, the patient carries a ventriculoperitoneal shunt (arrowhead). E. Chest X-ray at discharge. Complete clearing of lung opacities can be seen. Dilation of both pulmonary arteries is better recognized (arrows).
As physicians, we were prepared for COVID-19 catastrophic outcomes in CTEPH patients, based on their tampered haemostasis, delicate cardiorespiratory balance and comorbidities. However, the actual unfolding events surprised us with a relatively benign course. We considered four main physiopathological pathways potentially involved in this paradoxical behaviour: (1) reduced viral entrance to the pulmonary endothelium, (2) weakened ability to mount a severe inflammatory response, (2) dysregulation of pulmonary vasoactivity and (4) chronic anticoagulation to offset SARS-CoV-2 induced coagulopathy.
COVID-19 severity has been related to higher initial viral load.5 Angiotensin-converting enzyme 2 (ACE2) is the receptor to which SARS-CoV-2 binds to cross cell membrane.6 Reduced SARS-CoV-1 entrance has already been described in ACE2 knock-out mice.7 Interestingly, ACE2 expression is known to be reduced in the lungs of PAH patients.8 Although CTEPH-associated microvasculopathy shares many PAH-histological features, this reduced ACE2 expression has not been specifically described. However, decreased ACE2 expression has been seen in both PE patients and in animal models of thrombosis exhibiting higher thrombus volume.9 Therefore, reduced ACE2 expression would be expectable in CTEPH patients which may have contributed to reduce their initial viral load.
An initial adaptive immune response is necessary to eliminate the virus; however, once the lung endothelial cells are damaged, they induce innate inflammation which at this point becomes the leading cause for lung destruction. Levels of cytokines increase in this “second phase” and immune-modulating drugs become key in COVID-19 management.10 In this respect, endothelin-1, involved in CTEPH microvasculopathy, stimulates IL-6 secretion and treatment with ERAs may counteract this inflammatory trigger as already suggested in previous preclinical studies.11 Besides this, endothelial dysfunction has gained attention as a crucial factor for inflammation and microthrombosis; so, treatments with stabilizing properties have been considered beneficial. In this regard, PAH-specific medication besides ERA might add endothelial protection through NO release and antithrombotic-associated properties as in the case of prostacyclins.12
Loss of hypoxic vasoconstriction have been shown to be involved in the early stage of COVID-19 associated-ARDS, and may explain the severe hypoxaemia observed in these patients with relatively preserved compliance.13 In the CTEPH population, the already impaired pulmonary vasoactivity, prone to vasoconstriction, may prevent an increased perfusion in the non-ventilated areas; thus, this pathogenic mechanism.
Finally, haemostatic changes have been associated with both the initial lung insult and the clinical course in COVID-19. Experimental studies on SARS-CoV-1 showed an overexpression of prothrombotic and fibrinolytic factors that would increase vascular permeability and inflammation.14 Regarding SARS-CoV-2, histologic studies on COVID-19 specimens showed signs of thrombotic microangiopathy along with features of diffuse alveolar damage, highlighting the role of coagulopathy in COVID-19.4 Currently, venous thromboembolic prophylaxis is recommended in nearly all COVID-19 hospitalized patients and discussion on the necessity of weight-adjusted doses or full anticoagulation prescription is still ongoing.15 As common practice in CTEPH management, patients were already on chronic anticoagulation, which may have contributed to the benevolent course.
This work was supported by Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation (COV20/00181) – co-financed by European Development Regional Fund “A way to achieve Europe”.