Asthma currently affects 6–12% of the population in developed countries and it is estimated that affects 320 million people.1 Although asthma has been classically recognized as a disease associated with atopy and/or allergic disease, it is now considered a heterogeneous and multifactorial disease that encompasses different phenotypes and endotypes.2 Improvement of the knowledge related to the molecular pathways regarding the development and evolution of asthma has contributed to highlighting the relevance of establishing the inflammatory phenotype of asthmatic patients. In fact, the inflammatory phenotype prevails in the classifications used in most documents and clinical practice guidelines.3 Thus, asthma is classified based on the predominance (or absence) of the eosinophil-mediated T2 inflammatory pathway, which may be activated by allergic mechanisms (Th2) or by non-allergic mechanisms through type 2 innate lymphoid cells (ILC2). Taking this into account, current therapeutic strategies, especially in severe forms of the disease, focus on categorizing patients based on their inflammatory phenotype (T2 High vs. T2 low) in order to establish specific treatments targeting IgE, interleukin IL-5, IL-5 receptor, (IL)-4 receptor alpha subunit, and thymic stromal lymphopoietin (TSLP).
Despite these recent advances on the prevention of asthma exacerbations by approximately 50% based on drugs that block inflammatory pathways, recurrent disease exacerbations remain a major problem.4 Since the 1970s, it is well known that viral respiratory infections are the most common triggers for exacerbations of asthma, with human rhinoviruses (RV), particularly A and C, being the most frequent.5 Between 50 and 80% of asthma exacerbations in adults are linked to viral infections6,7 and RV is strongly associated with the return to school and the ‘September Epidemic’ in children.8 In this context, the relationship between asthma exacerbations (AE) and viral infections has raised some important questions. Do patients with a T2 inflammatory phenotype present more AE of viral origin? Which molecular mechanisms are involved in these exacerbation episodes? Are the current treatments for asthma effective for managing these episodes?
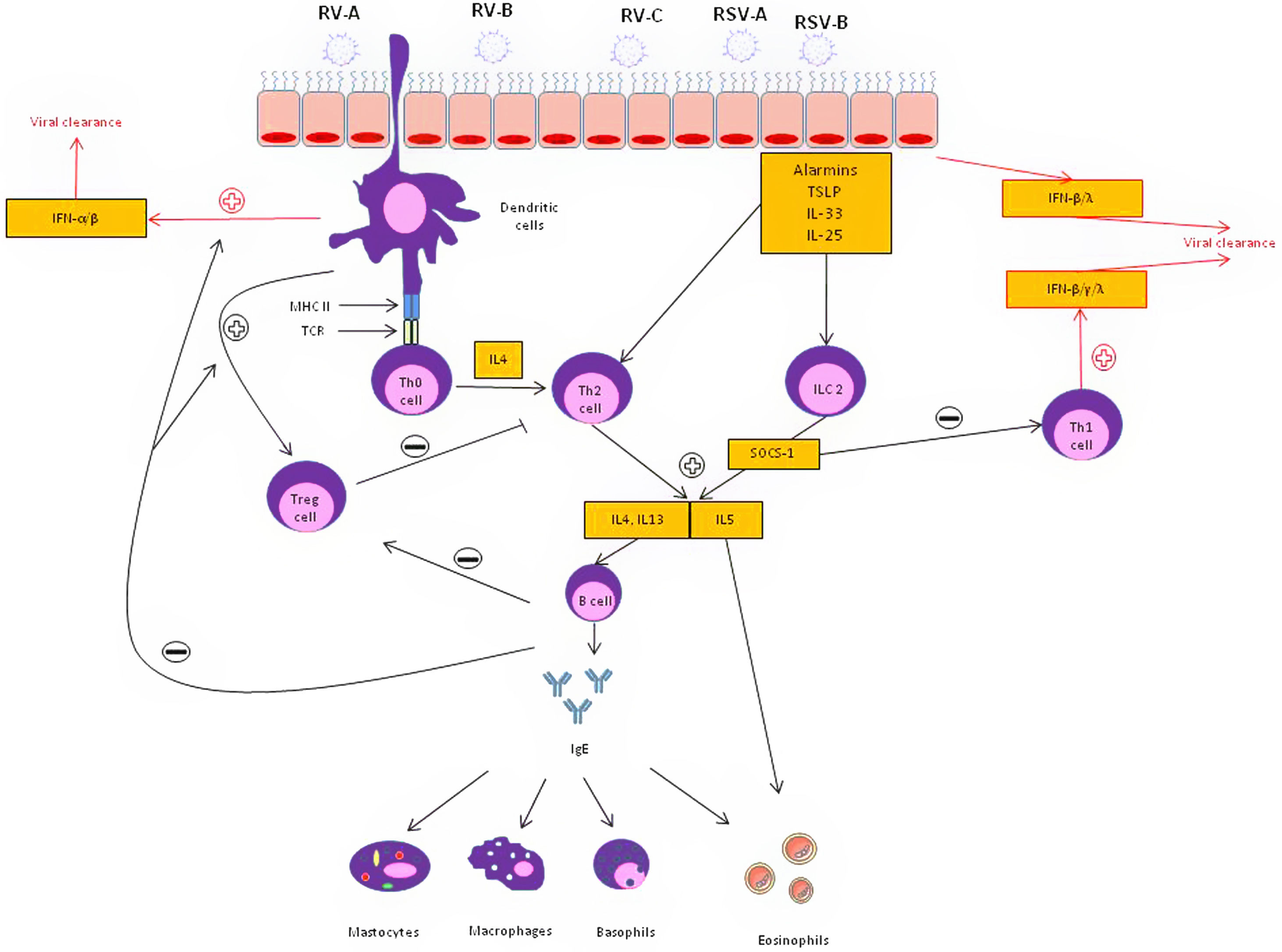
Regarding the first question, it is likely that patients with asthma presenting with a T2-high inflammatory phenotype are at higher risk of suffering viral AEs. Respiratory viral infections provoke a Th1 inflammatory response to promote viral clearance in the healthy host by the release of specific cytokines such as interferon (IFN)-γ, IFN-β and IFN-λ. These interferons are also responsible for preventing the entry and proliferation of viruses. Experimental studies with rhinoviruses in patients with asthma have shown a decrease in Th1 and IL10 type cytokines, which are associated with a protective effect against viral AE, and an increase in Th2 type cytokines, which are associated with more severe AE.9 This is supported by evidence demonstrating bronchial epithelial cells (BEC) from patients with asthma cultured with rhinovirus in vitro show lower IFN-β production and higher viral replication compared with healthy controls.10 In addition, when the BECs of asthmatic patients were treated with exogenous IFN-β, the rate of virus release was lower. Beyond an impaired IFN response, diverse mechanisms may be involved in the upregulation of T2 inflammatory response: (1) Respiratory viruses weaken the functional barrier of the airway epithelium, causing greater exposure to allergens and irritants capable of activating T2 inflammatory pathways. (2) Experimental RV inoculation induces the release of IL-25, IL-33 and TSLP from the airway epithelium of patients with asthma.11 These findings emphasize the relevance of the T2 inflammatory pathway as an important mechanism in AE of viral origin.
Despite the leading role of the T2 pathway, other inflammatory mechanisms are also possibly involved. Several authors have observed that rhinovirus infection activates the production of IL-8, stimulating the release of neutrophils and bronchial hyperresponsiveness.12 Neutrophils, in turn, activate elastase release, which promote the release of dsDNA forming neutrophil extracellular traps (NETs), mucus production in the airways leading to bronchial obstruction.
Several aspects need to be considered regarding the effectiveness of the treatments available for the management of these virally mediated AEs. In the context of the development of new biological drugs, the treatment approach to patients with severe asthma has undergone important changes. Until the end of the first decade of this century, oral corticosteroids were the only therapeutic alternative in patients with severe refractory asthma, regardless of the characteristics of the underlying inflammation. However, the arrival of new monoclonal biologics directed against different targets of the inflammatory cascade (anti-IgE, anti-IL-5, anti-IL-5 receptor, anti-TSLP, among others) have led to personalized and precision medicine in this disease. In this sense, it has been speculated that the biological drugs used for severe asthma, which significantly reduce the number of AE, could have a powerful antiviral effect. Since biological treatments as add-on therapy substantially reduce AEs in severe patients, and since more than 50% of AE are viral in origin, it seems logical that these treatments could have an impact on decreasing viral AEs. Several studies have described this effect with omalizumab and mepolizumab in the context of RV-mediated infections.13,14 Efthimiou et al.14 recently speculated that blocking IgE cross-linking with omalizumab, could up-regulate the recruitment of plasmacytoid cells, resulting in an activation of IFN-α and IFN-β, thus promoting viral clearance. In parallel, anti-IL-5 and anti-IL-5 receptor drugs could block suppressor of cytokine signaling 1 (SOCS-1), a T2 cytokine inductor that blocks Th1 response, leading to the up-regulation of Th1 response and the release of IFN-α, IFN-β, IFN-γ, IFN-λ and CXCL10 in the context of viral infection. Anti-IL4 receptor alpha(dupilumab) inhibits both the IL-4 and IL-13 pathway reducing both the activation and migration of eosinophils into the airways but also globet cell hyperplasia, leading to less mucus and thus less airway obstruction. Anti-TSLP (tezepelumab) treatment could also develop a relevant role in viral exacerbations by inhibiting the release of alarmins by epithelial cells and reducing a T2-ILC2 inflammatory response against the virus (Fig. 1).15
Epithelial and immune cell responses to viral infection in asthmatic patients. Following infection, a wide range of mediators are secreted leading to a Type 2 pathway upregulation through T-helper type 2(Th2) and type 2 innate lymphoid cell (ILC2) inflammatory mechanisms. In response to T2 inhibitor therapy, there is an up regulation of T-helper type 1 (Th1) immune pathway which is associated with increased interferon (INF). IL: interleukin; SOCS: suppressor of cytokine signaling; respiratory syncytial virus: RSV; rhinovirus: RV; T reg: T regulatory cells; TSLP: thymic stromal lymphopoietin.
Understanding the interaction between respiratory viruses and asthma will help develop a more precise targeted immunological approach for treating virally mediated AE. This can benefit each patient individually and, thus, reduce the deleterious interactions between respiratory virus and asthma: (1) exposure to respiratory viruses is associated with worsening asthma morbidity, (2) this interaction may be induced by different cellular structures and mechanisms, (3) there is a growing unmet need toward personalized or precision medicine, and (4) biological drugs aimed at inhibiting or regulating specific cellular response pathways involved in these interactions are being developed.
Conflicts of interestIO has received travel grants, consulting fees, talk fees or research grants from Astrazeneca, Bial, Boehringuer-Inguelheim, Chiesi, MSD, GlaxoSmithKline, Menarini, Mundipharma, Novartis, Puretech, TEVA and SANOFI. I.S. has received an ERS Respire 3 Marie-Curie Fellowship Award; research grants from GSK, Merck, personal fees for talks, consulting fees from GSK, Merck, AstraZeneca, Genentech and Respiplus outside the submitted work; OSU reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, and GlaxoSmithKline, personal fees from Cipla, Covis, Deva, Kamada, Kyorin, Menarini, Mereo Biopharma, Mundipharma, Napp, Novartis, Orion, Sandoz, Takeda, Trudell Medical, and UCB, and grants from Edmond Pharma, all of which are outside the submitted work.
IO is a researcher supported by the Pla Estratègic de Recerca i Innovació en salut (PERIS) 2016-2020 (SLT008/18/00108; G60594009). I.S. is current supported by the E.J.Moran Campbell Early Career Award, Department of Medicine, McMaster University.