In 2021, the Spanish Chronic Obstructive Pulmonary Disease (COPD) guidelines (GesEPOC 2021)1 were revised, modifying the definition of clinical phenotypes used to determine the initial pharmacological treatment of high-risk patients, compared to the previous edition.2 This classification was designed to tailor treatment to maximize disease control, especially with regard to the prevention of future adverse events, and in particular exacerbations and mortality. A basic premise is that risk group classification should be associated with a different incidence of adverse effects, but this principle has not yet been demonstrated for the new classification. The objective of this study was to determine the relationship between the risk group/phenotype classification of GesEPOC 2021 and the risk of future adverse events in a clinical cohort.
A retrospective study was conducted in patients attending a specialist COPD clinic at a university hospital (2008-September 2021). The inclusion criterion was diagnosis of COPD according to GesEPOC.1 Exclusion criteria were coexistence of respiratory pathology other than COPD (e.g., interstitial lung disease, pneumoconiosis), human immunodeficiency virus infection, alpha-1-antitrypsin deficiency, and non-availability of any parameter essential for classifying the individual according to GesEPOC. In particular, exacerbators who did not have at least 3 complete blood counts performed while stable in the previous 5 years were excluded. The index date was the date of the first outpatient clinic visit. The outcome variables were the incidence of severe COPD exacerbations requiring hospital admission after the index date, or death from any cause. Patients were classified as low risk if they met all of the following assumptions at the index date: FEV1>50%, modified Medical Research Council functional grade 0-1/4, and <2 moderate exacerbations and <1 severe exacerbation in the previous year. High-risk patients were those who did not meet all these conditions, and were classified as an exacerbator or non-exacerbator phenotype according to the aforementioned criteria.1 Following the methodology of a previous study, we classified high-risk exacerbators as eosinophilic if they had ≥300eosinophils/μl in at least 1 blood count performed while stable in the previous 5 years; all others were classified as non-eosinophilic.3 We performed an additional analysis in which an exacerbator was defined as eosinophilic when ≥300eosinophils/μl were detected in at least 3 of the blood counts performed in the previous 5 years. Differences between patient groups with respect to outcome variables were studied using Kaplan-Meier curves. This study is a secondary analysis of a study approved by the ethics committee of the Hospital Nuestra Señora de Candelaria (Ref: CHUNSC_2021_08).
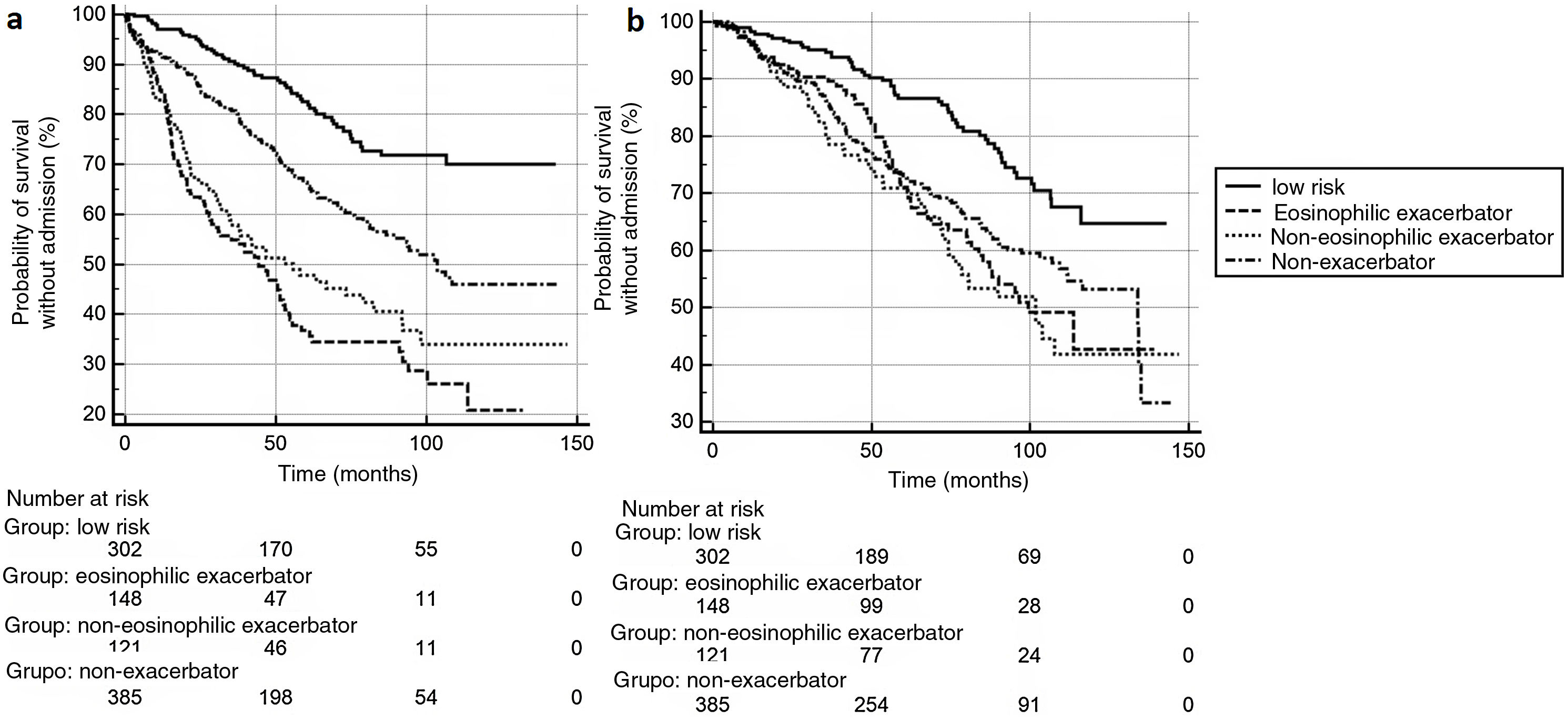
The eligible population comprised 1,047 consecutive subjects, included in a healthcare database. A total of 91 exacerbators who did not have at least 3 complete blood counts performed in the previous 5 years were excluded, leaving 956 study subjects. Mean age: 68.6±9.6 years. Men: 762 (79.7%). FEV1%: 52.8±17.0%. Mean follow-up: 66.7±36.2 months. Mortality: 302 cases (31.6%), within an average of 51.1±29.8 months after the index date. A total of 346 (36.2%) patients experienced≥1 severe exacerbation requiring admission. GesEPOC 2021 classification at the index date was: low-risk patients, 302 (31.6%); high-risk non-exacerbators: 385 (40.3%); high-risk eosinophilic exacerbators (using eosinophilia criteria from at least 1 blood count): 148 (15.5%); high-risk non-eosinophilic exacerbators: 121 (12.7%). Fig. 1 shows the Kaplan-Meier curves for (a) admissions for severe exacerbation and (b) mortality (p<0.001 for the comparison between curves in both cases). Table 1 shows the risk ratios for each GesEPOC classification group compared to the other groups, for both outcome events. The low-risk group showed a lower incidence of death than all high-risk groups (upper limit of the risk ratio confidence interval: <1 in all cases), with no significant differences among the latter groups. Differences were found among the different groups in the risk of severe exacerbations (Fig. 1a). Compared with the low-risk group, eosinophilic exacerbators had the highest incidence of exacerbations (risk ratio: 4.59; 95% CI: 3.23–6.51). However, when the eosinophilic exacerbator and non-eosinophilic exacerbator groups were compared, the differences did not reach statistical significance (Table 1). Moreover, in the additional analysis, in which the eosinophilic exacerbator group was defined as individuals with persistent eosinophilia (≥3 blood counts with ≥300 eosinophils), no significant differences were detected between this phenotype and the non-eosinophilic exacerbator (risk ratio: 0.57; 95% CI: 0.30–1.05).
Risk ratios and 95% confidence intervals for each GesEPOC classification group compared to the other groups*, for both outcome events (*column/row).
| Admission due to severe exacerbation | Death | |||||||
|---|---|---|---|---|---|---|---|---|
| GesEPOC group | Low risk | Eosinophilic exacerbator | Non-eosinophilic exacerbator | Non-exacerbator | Low risk | Eosinophilic exacerbator | Non-eosinophilic exacerbator | Non-exacerbator |
| Low risk | – | 4.59 (3.23–6.51) | 3.65 (2.53–5.27) | 2.07 (1.63–2.64) | – | 2.10 (1.47–2.98) | 2.40 (1.64–3.52) | 1.83 (1.40–2.40) |
| Eosinophilic exacerbator | 0.21 (0.15–0.30) | – | 0.79 (0.51–1.23) | 0.45 (0.32–0.63) | 0.47 (0.33–0.67) | – | 1.14 (0.74–1.76) | 0.87 (0.62–1.22) |
| Non-eosinophilic exacerbator | 0.27 (0.18–0.39) | 1.25 (0.80–1.94) | – | 0.56 (0.39–0.81) | 0.41 (0.28–0.60) | 0.87 (0.566–1.34) | – | 0.76 (0.52–1.10) |
| Non-exacerbator | 0.48 (0.37–0.61) | 2.20 (1.56–3.11) | 1.76 (1.22–2.52) | – | 0.54 (0.41–0.71) | 1.14 (0.81–1.59) | 1.30 (0.90–1.88) | – |
Our study confirms that clinical outcomes differ among the different groups of future risk classification proposed by GesEPOC 2021 and supports the clinical utility of this system. The most significant distinction, in terms of mortality, is the differentiation between low- and high-risk groups, but classification by phenotype does not appear to have a clear impact among high-risk patients. The GesEPOC classification was generally more useful for differentiating groups according to the risk of exacerbation, but failed to show a clear ability to predict events between the 2 exacerbator groups (eosinophilic and non-eosinophilic), although the visual analysis of Kaplan–Meier curves suggests that both behave differently. The study has several limitations in this regard: the statistical power may be insufficient to differentiate among groups of exacerbators; we only analyzed the risk of severe exacerbations requiring admission, but we did not examine moderate exacerbations, which also have a clinical impact on patients; more importantly, due to the long follow-up time and retrospective nature of the study, we were unable to analyze the influence that the different therapies may have had on the outcome variables. Finally, our definitions of the eosinophilic group were based on unvalidated historical values over a long period. It should be noted that previous studies have found that eosinophil values may vary over time,4 possibly affecting a long-term clinical outcome analysis, such as ours.5 Moreover, the clinical relevance of a classification based on a single eosinophil cut-off value is not fully clarified. A recent study in asthma found that the variability of this eosinophil figure over time is more indicative of a risk of severe exacerbation than the absolute cut-off points currently used in clinical decision-making.6 The value of this variable for predicting risk in COPD is still unknown.
Despite these limitations, the results of this study suggest that the GesEPOC classification for recommending drug treatments helps differentiate groups with different risks of future adverse events, both between low- and high-risk patients and among high-risk patients by comparing the non-exacerbators with exacerbators. It is, as such, a useful tool for tailoring COPD treatment.
Conflict of interestsOur study did not receive funding.