Lung transplantation (LT) for pulmonary fibrosis is related to higher mortality than other transplant indications. We aim to assess whether the amount of anterior mediastinal fat (AMF) was associated to early and long-term outcomes in fibrotic patients undergoing LT.
MethodsRetrospective analysis of 92 consecutive single lung transplants (SLT) for pulmonary fibrosis over a 10-year period. AMF dimensions were measured on preoperative CT-scan: anteroposterior axis (AP), transverse axis (T), and height (H). AMF volumes (V) were calculated by the formula: AP×T×H×3.14/6.
According to the radiological AMF dimensions, patients were distributed into two groups: low-AMF (V<20cm3) and high-AMF (V>20cm3), and early and long-term outcomes were compared by univariable and multivariable analyses.
ResultsThere were 92 SLT: 73M/19F, 53±11 [14–68] years old. 30-Day mortality (low-AMF vs. high-AMF): 5 (5.4%) vs. 15 (16.3%), p=0.014. Patients developing primary graft dysfunction within 72h post-transplant, and those dying within 30 days post-transplant presented higher AMF volumes: 21.1±19.8 vs. 43.3±24.7cm3 (p=0.03) and 24.4±24.2 vs. 56.9±63.6cm3 (p<0.01) respectively. Overall survival (low-AMF vs. high-AMF) (1, 3, and 5 years): 85%, 81%, 78% vs. 55%, 40%, 33% (p<0.001).
Factors predicting 30-day mortality were: BMI (HR=0.77, p=0.011), AMF volume (HR=1.04, p=0.018), CPB (HR=1.42, p=0.002), ischaemic time (HR=1.01, p=0.009).
Factors predicting survival were: AMF volume (HR=1.02, p<0.001), CPB (HR=3.17, p=0.003), ischaemic time (HR=1.01, p=0.001).
ConclusionPreoperative radiological assessment of mediastinal fat dimensions and volumes may be a useful tool to identify fibrotic patients at higher risk of mortality after single lung transplantation.
El trasplante de pulmón (TP) para el tratamiento de la fibrosis pulmonar está relacionado con una mayor mortalidad que otras indicaciones de trasplante. Nuestro objetivo es evaluar si la cantidad de grasa mediastínica anterior (GMA) se asoció a los diferentes resultados tempranos y a largo plazo en pacientes con fibrosis a los que se les realizó un TP.
MétodosAnálisis retrospectivo de 92 trasplantes de pulmón unilaterales (TPU) consecutivos para el tratamiento de la fibrosis pulmonar durante un período de 10 años. Se midieron las dimensiones de la GMA en la TC preoperatoria: eje anteroposterior (AP), eje transversal (T) y altura (A). Los volúmenes de GMA (V) se calcularon mediante la fórmula: AP×T×A×3,14/6.
Según las dimensiones radiológicas de la GMA, los pacientes se distribuyeron en 2 grupos: GMA baja (V<20cm3) y GMA alta (V>20cm3), y los resultados tempranos y a largo plazo se compararon mediante análisis univariables y multivariables.
ResultadosSe realizaron 92 TPU: 73V/19M, 53±11 (14-68) años. Mortalidad a 30 días (GMA baja frente a GMA alta): 5 (5,4%) frente a 15 (16,3%); p=0,014. Los pacientes que desarrollaron disfunción precoz del injerto dentro de las 72h posteriores al trasplante, y los que murieron dentro de los 30 días posteriores al trasplante presentaron mayores volúmenes de GMA: 21,1±19,8 frente a 43,3±24,7cm3 (p=0,03) y 24,4±24,2 frente a 56,9±63,6cm3 (p<0,01), respectivamente. Supervivencia global (GMA baja frente a GMA alta) (a los 1, 3 y 5 años): 85, 81 y 78% frente al 55, 40 y 33% (p<0,001), respectivamente.
Los factores que predijeron la mortalidad a los 30 días fueron: IMC (HR=0,77; p=0,011), volumen de la GMA (HR=1,04; p=0,018), CEC (HR=1,42; p=0,002), tiempo de isquemia (HR=1,01; p=0,009).
Los factores que predijeron la supervivencia fueron: volumen GMA (HR=1,02; p<0,001), CEC (HR=3,17; p=0,003) y tiempo de isquemia (HR=1,01; p=0,001).
ConclusiónLa evaluación radiológica preoperatoria de las dimensiones y los volúmenes de la grasa mediastínica puede ser una herramienta útil para identificar a aquellos pacientes con fibrosis con mayor riesgo de mortalidad después de un trasplante pulmonar único.
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive lung disease with a poor prognosis and a median survival of 3.8 years from the time of diagnosis.1 Despite the recent introduction of antifibrotic agents (nintedanib and pirfenidone), that have slowed down the rate of lung function decline, lung transplantation (LT) is the unique therapeutic modality that has demonstrated to improve survival for IPF patients, decreasing the risk of death by 75%.2
According to the most recent report of the ISHLT Registry, post-transplant median survival for IPF patients is 5.2 years, which is significantly worse than other LT indications, with higher rates of 30-day mortality, primary graft dysfunction (PGD), and longer hospital stay.3 Given the heterogeneity of IPF and the difficult prediction of the course of the disease, a careful selection of recipients with an appropriate assessment of postoperative risks is imperative. This makes necessary the establishment of accurate prediction models.
Even though IPF only affects lung parenchyma, the presence of an increased amount of fat in the anterior mediastinum has been described as a common finding in this disease. This is supported by the idea that mediastinal fat adapts to changes in lung volumes, as it has been also described after lobectomies or lung irradiation.4
The role of fat tissue in the pathogenesis of other diseases is well documented. Pericardial fat has been associated with metabolic risk factors and the severity of coronary artery disease, through the release of pro-inflammatory mediators.5 In addition, it has been suggested that higher volumes of epicardial fat tissue are associated with the severity of sclerodermia, regardless of other risk factors of cardiovascular disease or pulmonary fibrosis, hypothesizing that the proximity of the mediastinal fat to the pulmonary artery may exert pro-inflammatory effects contributing to disease progression.6
Translating this into the patient with IPF, we hypothesized that anterior mediastinal fat (AMF) may have an influence in the prognosis of the disease, irrespective of the changes in the pulmonary parenchyma. We then sought to determine whether the amount of AMF in fibrotic patients, candidates for LT, could be used as a surrogate marker of severity of disease, and therefore predict poor outcomes after the transplant procedure.
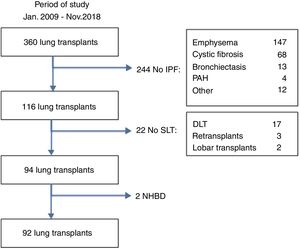
MethodsStudy designThe medical records from the pulmonary transplantation database of 360 LT patients transplanted between January 2009 and November 2018 at our Institution were retrospectively reviewed. Ninety-two patients with pulmonary fibrosis receiving a single lung transplant from brain-death donors were selected for the study (Fig. 1). Fibrotic patients fulfilled the general accepted criteria for lung transplantation.7 Patients were divided into two groups according to the amount of AMF volume, calculated on the basis of radiological measurements obtained from the preoperative chest CT-scan: low-AMF (volume <20cm3) and high-AMF (volume >20cm3). The present study followed the WMA Declaration of Helsinki-Ethical Principles for Medical Research involving human subjects. All patients signed the Informed Consent at the time of inclusion in waiting list and our Institutional Review Board approved this study.
Lung transplantation procedureLungs were retrieved from brain death donors using our standard protocol of cardiopulmonary harvesting previously reported.8 Either a right or left single LT was performed in all cases through a standard posterolateral thoracotomy or through an anterior thoracotomy. The surgical procedure and the postoperative standard of care for LT recipients were performed as previously reported by our Institution.9
Imaging analysisA pre-transplant chest CT-scan with intravenous contrast was performed in all patients, as part of the routine assessment of LT candidates. Scans were obtained at 10mm intervals in the supine position at end inspiration. The CT scan Digital Imaging Communications in Medicine (DICOM) format was used for assessment of axes and AMF volume.
A standardized manual tracing on the chest CT-scan was performed by two independent readers, blinded to each other. The radiological measurements of AMF included:
- -
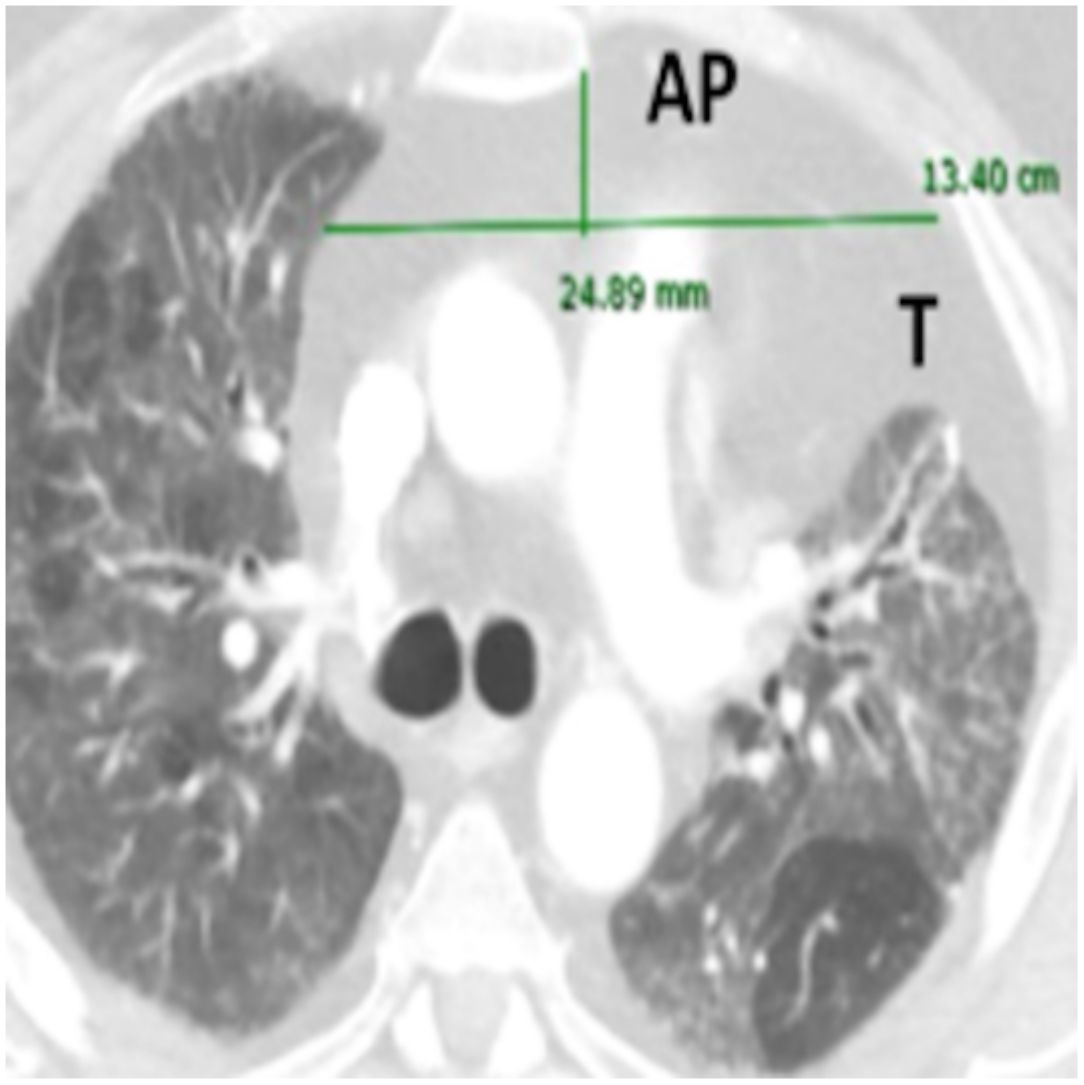
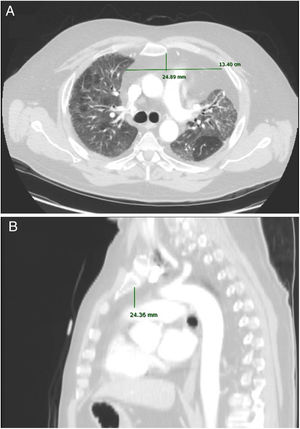
Anteroposterior axis (AP): from the posterior wall of sternum to the anterior wall of pulmonary artery, measured in the CT-axial plane (Fig. 2).
- -
Transverse axis (T): distance between both mediastinal pleurae, obtained at the same level of AP axis, and measured in the CT-axial plane (Fig. 2).
- -
Height (H): from posterior wall of the sternal notch to the main pulmonary artery trunk, measured in the sagittal plane (Fig. 2).
AMF volumes (V) were calculated by using the formula: AP×T×H×3.14/6, following a similar method to calculate prostatic volumes.10
Data collectionDonor data included those variables required to define optimal vs. extended donors. Recipient preoperative data included: age, gender, need of ventilation at the time of LT, body mass index (BMI), mean pulmonary artery pressure measured at induction of anaesthesia through a Swan-Ganz catheter, Lung Allocation Score (LAS) calculated at the time of LT as a surrogate marker of severity of illness, and radiological measurements of AMF (AP axis, transverse axis, height and volume).
Surgical and immediate postoperative data included: use of intraoperative extracorporeal life support (ECLS) (CPB or ECMO), ischaemic time, duration of mechanical ventilation, primary graft dysfunction (PGD) at 72h post-transplant, ICU stay, and 30-day mortality.
Late postoperative data included: overall mortality, airway complications, and survival.
Lung Allocation Score was calculated by using the Eurotransplant LAS calculator (www.eurotransplant.org, accessed: December 5, 2018).
DefinitionsPatients were diagnosed of idiopathic pulmonary fibrosis based on a pathological pattern of usual interstitial pneumonia and/or the presence of a typical radiological pattern on a high resolution CT-scan. In the absence of typical radiological findings, a lung biopsy was performed in all cases, excluding those patients with other interstitial lung diseases.
Recipients were considered on preoperative steroid therapy when receiving steroids at doses above 15mg/kg for 30 days just before transplantation.
Extended donors were defined as those having, at least, two of the following criteria: (1) age >55 years, (2) PaO2/FiO2 <350mmHg on 100% oxygen with 5cm H2O positive end-expiratory pressure, (3) pulmonary infiltrates on chest radiograph, (4) presence of purulent secretions on bronchoscopy, (5) ischaemic times >9h, and (6) tobacco history greater than 20 pack-years.
Primary graft dysfunction (PGD) was defined following the Statement of ISHLT.11 PGD grades 2–3 at 72h post-transplant were included in the analysis.
Airway complication was defined as a finding of dehiscence, stenosis, or malacia of the anastomosis requiring either intervention (surgery, dilatation, debridement, laser therapy or stent placement) or only conservative measures.
Urgent lung transplants were defined as those performed in candidates listed for transplant with rapid clinical deterioration requiring preoperative invasive mechanical ventilation at the time of the procedure.
Statistical analysisThe inter-reader agreement of radiological measurements was assessed by the Pearson correlation of the means of the two readers, and by the intra-assay coefficient of variation.
30-Day mortality and survival were compared by univariable and multivariable analyses (logistic regression and Cox models) adjusting for recipient age, LAS, BMI, use of extended donor, use of ECLS, ischaemic time, and PGD.
A univariable analysis comparing low AMF vs. high AMF was performed first: either Pearson's χ2 or Fisher's exact test was used, when appropriate, to assess differences between categorical variables.
Unpaired t-test was used to compare means between two quantitative variables from normally distributed data, and Mann–Whitney U-test for non-normally distributed data. Given the large sample size of the study population, we assumed homogeneous variances and normal distribution in most of the analyses. We used parametric tests when more than 30 cases for each group were compared and non-parametric tests when less than 30 cases for each group were compared. Pre-tests for normality were not performed.
The relationship between radiological measurements and some demographic and functional variables was assessed by the Pearson correlation coefficient.
Survival was analyzed and compared using the Kaplan–Meier method and log-rank test.
To determine independent predictors of mortality, those variables exhibiting p values below 0.1 in the univariable analyses entered into a multivariable Cox-regression analysis (forward stepwise likelihood ratio). Those variables with p values below 0.05 in the final model were judged to be independent predictors of mortality.
Continuous variables are expressed as means±standard deviation. Categorical variables are expressed as counts and proportions with 95% confidence intervals (95% CI). Differences with p values <0.05 were considered significant. The statistical analysis was performed using SPSS (SPSS 20.0 for Mac: SPSS, Inc., Chicago, IL, USA).
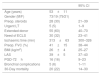
ResultsOverall patient seriesThere were 92 patients, 73 males (79%), and 19 females (21%), 53±11 [14–68] years old. Twenty-eight patients (30%) were under preoperative steroid therapy. Overall AMF measurements were: AP 22±10 [7–65]mm, T 67±28 [7–150]mm, H 33±13 [9–70]mm, V 31.55±38.54 [4.61–274.75]cm3. AMF measurements did not differ by gender or preoperative steroid therapy.
General data of the patient series are depicted in Table 1.
General description of patient series (n=92).
| 95% CI | ||
|---|---|---|
| Age (years) | 53±11 | |
| Gender (M/F) | 73/19 (79/21) | |
| Preop. steroids | 28 (30) | 21–39 |
| Urgent LT | 5 (5) | 1–9 |
| Extended donor | 55 (60) | 40–70 |
| Need of ECLS | 30 (32) | 23–41 |
| Ischaemic time (min) | 313±63 | 300–326 |
| Preop. FVC (%) | 41±15 | 38–44 |
| BMI (kg/m2) | 26±4 | 25–27 |
| LAS | 39±5 | 37–40 |
| PGD-72h | 16 (16) | 9–23 |
| Bronchial complications | 5 (6) | 1–11 |
| 30-Day mortality | 20 (22) | 14–30 |
BMI: body mass index; ECLS: extracorporeal life support (CPB or ECMO); FVC: forced vital capacity; LAS: Lung Allocation Score; LT: lung transplantation; PGD: pulmonary graft dysfunction at 72h postop. Quantitative variables are expressed as means±SD. Qualitative variables are expressed as counts and proportions, in parenthesis. BMI and LAS were calculated at the time of transplantation.
The inter-reader agreement for radiological measurements was excellent, both by Pearson coefficients of correlation (AP axis: r=0.98, p<0.001; T axis: r=0.94, p<0.001; height: r=0.97, p<0.001) and intra-assay coefficients of variation (AP axis: 0.08; T axis: 0.06; height: 0.12).
Comparative analysis between right and left lung transplantsTo elucidate whether the side of LT could affect overall outcomes, we compared right and left LT by univariate analysis. No differences were observed when right or left lungs were transplanted. Sixty-six patients underwent a right LT (60%) and 26 underwent a left LT (40%). Age (right vs. left LT): 53±9 vs. 52±1 years; p=0.64. Ischaemic time (right vs. left LT): 312±63 vs. 315±65min; p=0.89. Thirty-day mortality (right vs. left LT): 15 (23%) vs. 5 (19%); p=0.47. Primary graft dysfunction (right vs. left LT): 6 (9%) vs. 0; p=0.06. High/low AMF (right vs. left LT): 31/35 vs. 16/10; p=0.15. Need of ECLS (right vs. left LT): 12 (18%) vs. 5 (19%); p=0.56.
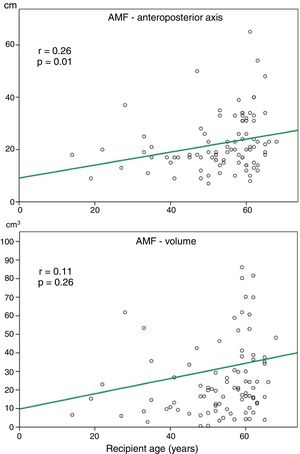
Relationship between AMF volume and demographic and functional parametersAlthough the amount of AMF could be influenced by some factors such as nutritional status, loss of respiratory function or recipient age, on a preliminary analysis, we did not observe a significant correlation between AMF volume and BMI (r=0.12, p=0.27), or AMF volume and FVC (%) (r=0.14, p=0.45). Although there was a trend towards more AMF volume in older recipients, this correlation was not significant (Fig. 3).
Relationship between AMF volume and recipient perioperative factorsAMF volumes did not differ between those patients receiving preoperative steroids (27.8±26.3cm3; 95% CI: 22.5–33.1cm3) and those who did not (35.1±52.1cm3; 95% CI: 24.5–45.7cm3). In addition, no differences in AMF volumes were observed in patients requiring urgent LT or those developing bronchial complications (data not shown).
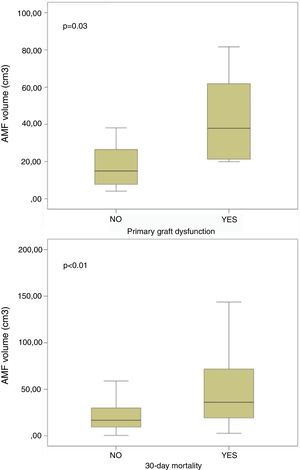
On the contrary, it is noteworthy that those patients developing PGD at 72h post-transplant, and those dying within 30 days post-transplant presented significant higher volumes of AMF pre-transplant (Fig. 4). These differences remained significant when grouping patients into low AMF volume and high AMF volume: 15 patients with high AMF died within 30 days post-transplant, as opposed to only 5 patients in the low-AMF group (p=0.014). In addition, the high AMF group required more frequently ECLS than the low-AMF group. Finally, in the high-AMF group, there was a tendency to be male, older patients, and with longer ischaemic times (Table 2).
Unadjusted comparative analysis of fibrotic transplant patients with low AMF volumes (<20cm3) vs. high AMF volumes (>20cm3).
| Low AMF(n=45) | 95% CI | High AMF(n=47) | 95% CI | p | |
|---|---|---|---|---|---|
| Recipient age (years) | 50±11 | 55±9 | 0.05 | ||
| Recipient gender | |||||
| Male | 32 (71) | 41 (87) | 0.04 | ||
| Female | 13 (29) | 6 (13) | |||
| Preop. steroids | 11 (24) | 12–36 | 17 (36) | 22–50 | 0.17 |
| Preop. FVC (%) | 40±18 | 35–45 | 42±11 | 39–45 | 0.70 |
| BMI (kg/m2) | 25±4 | 23–26 | 27±3 | 26–28 | 0.27 |
| Urgent LT | 3 (6) | 0–12 | 2 (4) | 0–8 | 0.48 |
| LAS | 39±1 | 38.7–39.3 | 41±2 | 40.5–41.5 | 0.28 |
| Extended donor | 22 (49) | 35–63 | 33 (70) | 57–83 | 0.06 |
| Need of ECLS | 8 (18) | 7–29 | 22 (47) | 33–61 | 0.01 |
| CPB | 0 | 3 (6) | 0–12 | ||
| ECMO | 8 (18) | 7–29 | 19 (40) | (34–46) | |
| Ischaemic time (min) | 301±52 | 286–316 | 325±71 | 305–345 | 0.07 |
| PGD-72h | 5 (11) | 2–20 | 11 (23) | 11–35 | 0.03 |
| Bronchial complications | 2 (4) | 0–8 | 3 (6) | 0–12 | 0.51 |
| Recipient ICU stay (days) | 10±15 | 6–14 | 10±11 | 7–13 | 0.87 |
| 30-Day mortality | 5 (11) | 2–20 | 15 (32) | 19–45 | 0.01 |
| Overall mortality | 10 (22) | 10–34 | 29 (62) | 48–76 | <0.01 |
BMI: body mass index; ECLS: intraoperative extracorporeal life support (CPB or ECMO); FVC: forced vital capacity; LAS: Lung Allocation Score; LT: lung transplantation; PGD-72h: primary graft dysfunction at 72h postop. Quantitative variables are expressed as means±SD. Qualitative variables are expressed as counts and proportions, in parenthesis.
Focusing on potential causes of 30-day mortality in an unadjusted analysis, we observed that patients dying within 30 days post-transplant were those in poor clinical condition, with low BMI and higher LAS, requiring an urgent LT under ECLS (with longer ischaemic times) (Table 3).
Univariable analysis of post-transplant 30-day mortality.
| 30-Day mortality | No(n=70) | 95% CI | Yes(n=22) | 95% CI | p |
|---|---|---|---|---|---|
| Recipient age (years) | 54±10 | 49±14 | 0.11 | ||
| Recipient gender | |||||
| Male | 59 (84) | 15 (68) | 0.19 | ||
| Female | 11 (16) | 7 (32) | |||
| Preop. steroids | 22 (31) | 20–42 | 6 (27) | 9–45 | 0.59 |
| Preop. FVC (%) | 41±15 | 37–44 | 37±12 | 32–42 | 0.62 |
| BMI (kg/m2) | 27±3 | 26–28 | 24±4 | 22–26 | 0.01 |
| Urgent LT | 0 | 5 (23) | 6–40 | <0.01 | |
| LAS | 35±2 | 34.5–35.5 | 39±3 | 37.8–40.2 | <0.01 |
| Extended donor | 41 (58) | 47–69 | 14 (63) | 42–83 | 0.17 |
| Need of ECLS | 9 (13) | 5–21 | 21 (95) | 86–100 | 0.01 |
| CPB | 3 | 0 | |||
| ECMO | 6 (8) | 2–14 | 21 (95) | 86–100 | |
| Ischaemic time (min) | 300±56 | 287–313 | 364±65 | 338–360 | <0.01 |
| PGD-72h | 9 (13) | 5–21 | 6 (27) | 9–45 | 0.54 |
| Bronchial complications | 5 (7) | 1–13 | 0 | 0.27 | |
BMI: body mass index; ECLS: intraoperative extracorporeal life support (CPB or ECMO); FVC: forced vital capacity; LAS: Lung Allocation Score; LT: lung transplantation; PGD-72h: primary graft dysfunction at 72h postop. Quantitative variables are expressed as means±SD. Qualitative variables are expressed as counts and proportions, in parenthesis.
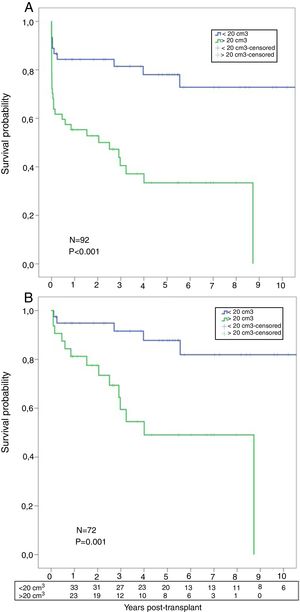
A significant worse post-transplant survival was observed in patients with higher AMF volumes. Actuarial survival for low-AMF patients was 85%, 81%, and 78% at 1, 3, and 5 years respectively, with a median survival of 8.3 years. On the contrary, actuarial survival for high-AMF patients was 55%, 40%, and 33% at 1, 3, and 5 years respectively, with a median survival of 2.5 years (p<0.001) (Fig. 5A). When we analyzed the survival, conditional to survive 30 days, these differences remained significant: 95%, 91%, 88% at 1, 3, and 5 years in the low-AMF group vs. 81%, 59%, 49% at 1, 3, and 5 years in the high-AMF group (p=0.001) (Fig. 5B).
(A) Post-transplant comparative survival between fibrotic patients with low AMF volumes vs. patients with high AMF volumes. (B) Post-transplant comparative survival between fibrotic patients with low AMF volumes vs. patients with high AMF volumes among those surviving 30 days post-transplant.
Factors predicting 30-day mortality were BMI (HR=0.77, p=0.011), AMF volume (HR=1.04, p=0.018), ECLS (HR=1.42, p=0.002), and ischaemic time (HR=1.01, p=0.009).
Factors predicting survival were AMF volume (HR=1.02, p<0.001), ECLS (HR=3.17, p=0.003), and ischaemic time (HR=1.01, p=0.001) (Table 4).
Factors predictive of 30-day mortality and survival by multivariable analysis.
| HR | 95% CI | p | |
|---|---|---|---|
| 30-Day mortality | |||
| BMI | 0.77 | 0.64–0.91 | 0.011 |
| AMF volume | 1.04 | 1.02–1.05 | 0.018 |
| ECLS | 1.42 | 1.26–1.68 | 0.002 |
| Ischaemic time (min) | 1.01 | 1.0–1.3 | 0.009 |
| Survival | |||
| AMF volume | 1.02 | 1.01–1.04 | <0.001 |
| ECLS | 3.17 | 2.61–3.77 | 0.003 |
| Ischaemic time (min) | 1.01 | 1.0–1.03 | 0.001 |
AMF: anterior mediastinal fat; BMI: body mass index; ECLS: extracorporeal life support; HR: hazard ratio.
In the present study, we were able to demonstrate that the AMF volume in IPF patients may play a role in predicting poor outcomes after LT. Patients with high AMF volumes presented more 30-day mortality and poorer survival than those with low AMF volumes, both in univariable and multivariable analyses.
To our knowledge, this is the first report aiming to analyze the volume of mediastinal fat tissue in fibrotic patients and its potential use as a surrogate marker of severity of disease, and therefore, as an individual risk factor in patients undergoing LT.
For the purposes of this study, we selected a homogeneous group of IPF patients undergoing single LT. Even though bilateral LT have demonstrated better survival than single LT in IPF,3 in our Centre, most of fibrotic patients underwent single procedures on the basis of optimizing the scarcity of donor resources.12
Parenchymal radiological findings of the chest CT scan play an essential role in the initial assessment and disease progression. On the other hand, some extraparenchymal radiological findings have been proposed as factors related to poor prognosis in IPF patients. Tanizawa et al.13 reported poor outcomes of LT in patients with pleuroparenchymal fibroelastosis. Others have suggested that an increased amount of AMF and lymph node enlargement are associated to IPF progression,14 but current clinical guidelines do not include them as diagnostic or prognosis factors. For this reason, we hypothesized that an increased amount of AMF could be a predicting factor of post-transplant outcomes in IPF patients, by making a simple measurement in the preoperative chest CT scan. The calculation of AMF volume was obtained by using the formula widely used and validated for calculation of volumetric values in other organs.10
Several factors, such as coronary artery disease, gastroesophageal reflux, telomerase disorder, and pulmonary hypertension have been associated with a poorer prognosis after LT in IPF patients.2,3,15 In addition, older recipients, low exercise capacity, poor functional parameters, and high oxygen needs are additional risk factors. To deal with an accurate method of patient severity of illness, we used the LAS score, calculated at the time of transplantation, to establish a composite value of severity of illness that includes, age, gender, anthropometric data, diabetes, ventilation needs, forced vital capacity, pulmonary artery pressures, PCO2 changes, 6-min walk distance and serum creatinine.16 In our Centre, LAS score is not used to establish a priority on waiting list for LT.
In our series, higher AMF volumes did not correlate with BMI values, steroid therapy or age, although there was a trend to higher AMF volumes in older recipients (Fig. 3). These findings might suggest that AMF changes in fibrotic patients are not influenced by these factors, although opposite findings have been reported regarding the development of AMF in patients receiving high doses of steroids.17 Regarding novel antifibrotic treatments of IPF, there is no evidence that nintedanib and pirfenidone could alter body fat distribution.1 In the present series, a few numbers of patients were given antifibrotics and thus, this parameter was not included in the analysis.
Although it has been suggested an association between AMF and IPF severity, by a negative correlation with FVC and DLCO,4 we did not observe such a relationship, possibly due to the subtle differences on AMF volumes and its small influence on lung volumes in the present series. Therefore, we could not demonstrate a fat substitution as a consequence of the lung loss of volume and elastic recoil in end-stage IPF patients.
Compared with other indications, IPF presents the worst early and long-term outcomes after LT.3 In the present series, patients dying within 30 days post-transplant were those in poor general condition, with low BMI, higher LAS, ventilated patients and need of ECLS (Table 3). These are well known risk factors that have been documented in the literature.3,19 To note that only 3 patients in the group of high AMF volume underwent CPB (6%), as opposed to 27 patients undergoing ECMO (94%) and none of CPB patients died within 30 days. Potential subtle biases by grouping these methods of ECLS might be minimized by the low number of patients under CPB. In the present series, we also observed that LT patients with higher AMF volumes presented more PGD episodes and higher rates of 30-day mortality, which suggest that AMF may be an additional risk factor of early pulmonary dysfunction and subsequent early mortality post-transplantation.
To sustain this observation, the adjusted risk model for 30-day mortality confirmed that AMF volume was independently associated to early mortality, in addition to other well-known risk factors such as BMI, need of ECLS or ischaemic times.3,18
IPF patients with high AMF volumes presented less survival than those with low AMF volumes. These differences might be influenced by other confounding factors such as higher LAS, PGD, longer ischaemic times or the need of ECLS.18 However, after the exclusion of those recipients dying within 30 days post-transplant, the differences remained significant. In addition, the AMF volume remained an independent predictor, as well as other risk factors such as the need of ECLS or longer ischaemic times. It is possible that patients with higher AMF volumes were those in poor general condition, requiring preoperative ventilation, intraoperative ECLS, with longer ischaemic times, and developing PGD more frequently post-transplantation.
The mechanisms involved in the possible deleterious effects of AMF on LT outcomes are unknown. It has been well documented that visceral abdominal and epicardial fat are related with the pathogenesis of cardiovascular and systemic adverse events through the release of pro-inflammatory mediators by different mechanisms.5,19 On one hand, abdominal mesenteric fat has a circulatory communication path to the liver via the portal circulation and it has been thus associated with hepatic production of inflammatory factors. On the other hand, intrathoracic and pericardial fat depots are substantially smaller than abdominal fat and are unlikely to release substances that could be detected systemically. Therefore, their role is more likely to be paracrine via their local effect on inflammation in the underlying tissue.19 Accordingly, it has been postulated that the close proximity of the epicardial fat tissue to the heart and vessels and the lack of a physical barrier between these structures allow for diffusion of pro-inflammatory cytokines that could play role in the development of cardiometabolic diseases.19
We hypothesize that the same local effects of epicardial fat tissue observed in patients with cardiac disease may be translated to the AMF for patients with IPF. The role of AMF as a potential local source of pro-inflammatory mediators that could play a role in the natural history of IPF remains to be investigated.
The present study has several limitations. First, the retrospective nature of this analysis. Second, the method used for the calculation of AMF volumes is imperfect. Obtaining radiological measures from a chest CT scan to calculate volumes is subject of observer variability. Despite the excellent inter-reader agreement between observers, some bias in determining the exact dimensions of AMF is expectable, and, possibly, other computed methods for assessing AMF values could have been desirable.20 In addition, the election of a cut-point of 20cm3 of AMF to distribute patients into low and high AMF was arbitrary, based on the median AMF values of the patient series. Third, there was a time-gap between the preoperative chest CT scan, from which AMF measurements were obtained, and the transplant procedure, from which the LAS was calculated, and it is possible that AMF volumes were underestimated in some severely ill IPF patients at the time of the LT. Fourth, we did not consider for the analysis some variables that could have had an impact on the results reported herein, such as preoperative use of antifibrotic agents, variations in the intraoperative anaesthetic management, duration of ECLS, the intraoperative use of blood products, etc. Finally, although we observed an association between AMF volumes and LT outcomes in fibrotic patients, we cannot demonstrate a cause-effect relationship. Furthermore, the possible mechanisms of this association remain unanswered.
In conclusion, we have observed that IPF patients with high volumes of AMF undergoing LT present poorer early and long-term outcomes than those with low volumes of AMF preoperatively. Although the pathogenesis of this association is unknown, we hypothesize that either a mediastinal fat substitution associated to the progressive lung volume reduction in IPF patients, a paracrine effect of AMF by releasing pro-inflammatory mediators in these patients, or both, may be potential mechanisms involved. Additional investigations are warranted to determine whether AMF has a role in predicting IPF progression and, subsequently, poor outcomes after LT.
Conflict of interestsThe authors declare no conflict of interest.