Invasive respiratory support is a cornerstone of Critical Care Medicine, however, protocols for withdrawal of mechanical ventilation are still far from perfect. Failure to extubation occurs in up to 20% of patients, despite a successful spontaneous breathing trial (SBT).
MethodsWe prospectively included ventilated patients admitted to medical and surgical intensive care unit in a university hospital in northern Mexico. At the end of a successful SBT, we measured diaphragmatic shortening fraction (DSF) by the formula: diaphragmatic thickness at the end of inspiration – diaphragmatic thickness at the end of expiration/diaphragmatic thickness at the end of expiration×100, and the presence of B-lines in five regions of the right and left lung. The primary objective was to determine whether analysis of DSF combined with pulmonary ultrasound improves prediction of extubation failure.
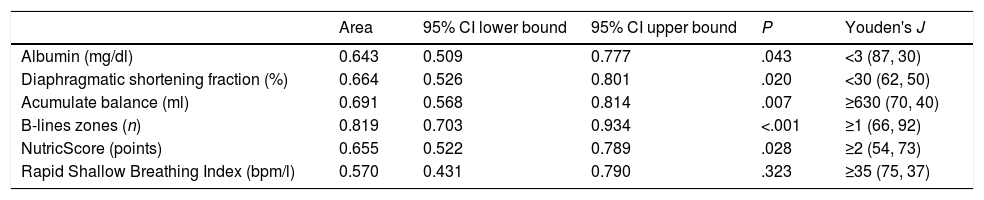
ResultsEighty-two patients were included, 24 (29.2%) failed to extubation. At univariate analysis, DSF (Youden's J: >30% [sensibility and specificity 62 and 50%, respectively]) and number of B-lines regions (Youden's J: >1 zone [sensibility and specificity 66 and 92%, respectively]) were significant related to extubation failure (area under the curve 0.66 [0.52–0.80] and 0.81 [0.70–0.93], respectively). At the binomial logistic regression, only the number of B-lines regions remains significantly related to extubation failure (OR 5.91 [2.33–14.98], P<.001).
ConclusionIn patients with a successfully SBT, the absence of B-lines significantly decreases the probability of extubation failure. Diaphragmatic shortening fraction analysis does not add predictive power over the use of pulmonary ultrasound.
El soporte respiratorio invasivo constituye una piedra angular en la medicina de cuidados intensivos. Sin embargo, los protocolos para retirar la ventilación mecánica todavía están lejos de ser perfectos. El fallo de extubación ocurre en hasta un 20% de los pacientes, a pesar del éxito en la prueba de respiración espontánea (SBT).
MétodosSe incluyeron de forma prospectiva pacientes con ventilación ingresados en una unidad médica y quirúrgica de cuidados intensivos de un hospital universitario del norte de Méjico. Tras el éxito en una SBT, se midió la fracción de acortamiento diafragmático (DSF) mediante la fórmula: (grosor diafragmático al final de la inspiración – grosor diafragmático al final de la expiración)/grosor diafragmático al final de la expiración × 100, y la presencia de líneas B en cinco regiones del pulmón derecho y del izquierdo. El objetivo primario fue determinar si el análisis de la DSF combinado con la ecografía pulmonar mejora la predicción del fallo de extubación.
ResultadosSe incluyeron 82 pacientes, 24 (29,2%) con fallo de extubación. En el análisis univariante, la DSF (Índice de Youden: >30% [sensibilidad y especificidad del 62% y el 50%, respectivamente]) y el número de regiones con líneas B (Índice de Youden: >zona 1 [sensibilidad y especificidad del 66% y el 92%, respectivamente]) se relacionó significativamente con el fallo de extubación (área bajo la curva 0,66 [0,52-0,80] y 0,81 [0,70-0,93] respectivamente). En la regresión logística binaria, solo el número de regiones con líneas B se relacionó significativamente con el fallo de extubación (OR 5,91 [2,33-14,98], p<0,001).
ConclusiónEn pacientes con éxito en la SBT, la ausencia de líneas B disminuye significativamente la probabilidad de fallo de extubación. La fracción de acortamiento diafragmático no añade valor predictivo respecto al uso de la ecografía pulmonar.
Mechanical ventilation (MV) is a cornerstone of the Critical Care Medicine. Although criteria for the initiation of invasive respiratory support are well defined, protocols for his withdrawal are still far from being perfect.1
Several parameters have been used to predict the optimum time of extubation; among the most used are the rapid shallow breathing index (RSBI), weaning index, maximal inspiratory pressure, vital capacity, spontaneous current volume and trans-diaphragmatic pressure, to name but a few. However, despite a careful selection of patients with tolerance to the decrease of ventilatory support, extubation failure still occurs in up to 20% of cases.2 Furthermore, several studies have shown that the need for reintubation within 48h after MV withdrawal is independently related to a prolonged hospital stay and mortality increase.
Recently, pulmonary, pleural and thoracic wall ultrasound has proven to be particularly useful in the field of pulmonary and critical care medicine.3–6 A particular interest is the use of ultrasound for the evaluation of two fundamental aspects of the process of MV release: diaphragmatic function and the estimation of extravascular lung water (EVLW). Being a non-invasive tool and not requiring the use of ionizing radiation, this technique offers advantages over the methods traditionally used for the evaluation of the lung and diaphragm.7,8
Ultrasonography measurements of the diaphragmatic shortening fraction (DSF) and the presence or absence of parenchymal B-lines are functional parameters easy to obtain. Timely identification of diaphragmatic dysfunction is desirable because, during spontaneous breathing trial (SBT), accessory respiratory muscles may be sufficient to achieve a RSBI without leading to successful extubation given its rapid fatigability. On the other hand, pulmonary ultrasound timely detects the increase of extravascular pulmonary water secondary to venocapillary pulmonary hypertension; an SBT with the appearance of B-lines consistently predicts extubation failure.9,10
The primary objective in this study was to evaluate whether the combination of thoracic ultrasound assessment of DSF and the number of lung zones with B-lines could improve the prediction of extubation failure in adults critical care patients with a successful SBT.
MethodsPatients and Ultrasound ProtocolOur hospital is a tertiary teaching center in northern Mexico; it has 23 intensive care beds. Between March 2016 and June 2017, we prospectively included patients >18 years of age admitted to medical or surgical intensive care unit (ICU) with a successful SBT, with support pressure and continuous positive airway pressure of zero cmH2O for 30min, as part of our regular weaning protocol. We excluded patients with previous extubation failure, injuries that prevent ultrasound realization, pregnancy, neuromuscular diseases, Glasgow coma scale<8 points, pneumothorax, right pleural tube or know diaphragmatic paralysis.
Diaphragmatic and lung ultrasound were performed by a senior resident of the pulmonary and critical care medicine (CPR) under the supervision of a professor with expertise in ultrasound performance (EJR). For the inter-observer variability analysis, another senior resident (ER) with the supervisions of a second professor (RM) perform a second blinded ultrasonography study immediately after the completion of the first one. Ultrasound was performed using the GE Logiq XP (SOMA Technology, Inc.) system equipped with a 10MHz linear probe. With the patient in a supine position, the diaphragm was visualized as two parallel echogenic lines at the eighth or ninth intercostal space at the mid-axillary line. We captured the images during inspiration and expiration at tidal volume and during maximal inspiration and expiration. Each image was frozen at B mode, and the diaphragmatic thickness was measured from the center of the pleural line to the middle of the peritoneal line. The diaphragmatic thickening fraction was calculated by the formula: diaphragmatic thickness at the end of the inspiration – diaphragmatic thickness at the end of the expiration/diaphragmatic thickness at the end of expiration ×100. For the lung ultrasound assessment, we analyzed the presence of B-lines in each of five regions of the right and left lung according to describe by the BLUE protocol.4
We defined extubation failure when the patient required endotracheal tube reinsertion in the 48h since extubation. A study-independent attending physician decided extubation and reintubation without the knowledge of ultrasound analysis results.
Sample Size and Statistic AnalysisUsing the formula for the diagnostic test: n=4(zα)2 (pq)/IC2, with a value zα of 1.96 with a 95% significance level for two tails, an expected sensitivity of 90%,11 and maximum amplitude of the confidence interval of 0.1, a sample of 82 participants was obtained. The sample size for the concordance analysis between observers calculated with the formula for the comparison of means (n=2σ2K/ɛ2), with a zα value of 1.96, significance level of 95% for two tails and 80% potency expecting to find an inter-observer difference of ±1mm was of 16 measurements.
We tested normal distribution with the Kolmogorov–Smirnov method. Data are shown as means and standard deviations for variables with normal distribution and as medians and interquartile ranges for non-normal variables. We used the Student's t-test, Mann–Whitney U-test, ANOVA, or chi-square as indicated. For the most significant variables at univariate analysis, we calculated the receiver operating characteristic curve, area under the curve, Youden's J statistic and correlations between variables by the Kendall's Tau method.
According to our sample size, we tested the five most significant variables in multivariate analysis by binomial logistic regression; we used the Box–Tidwell procedure with Bonferroni correction for the linearity assessment of the continuous variables with respect to the Logit of the dependent variable.
Concordance between different ultrasound operators was calculated by weighted Kappa with the quadratic approach.
We defined a statistically significant difference as a P value <.05. The analysis was performed using SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL).
EthicsPatient's legal guardian provided informed consent. The study was approved by the ethics committee of Dr. José E. González University Hospital (registration number NM16-00003) and was registered in ClinicalTrials.gov (registration number NCT02799056).
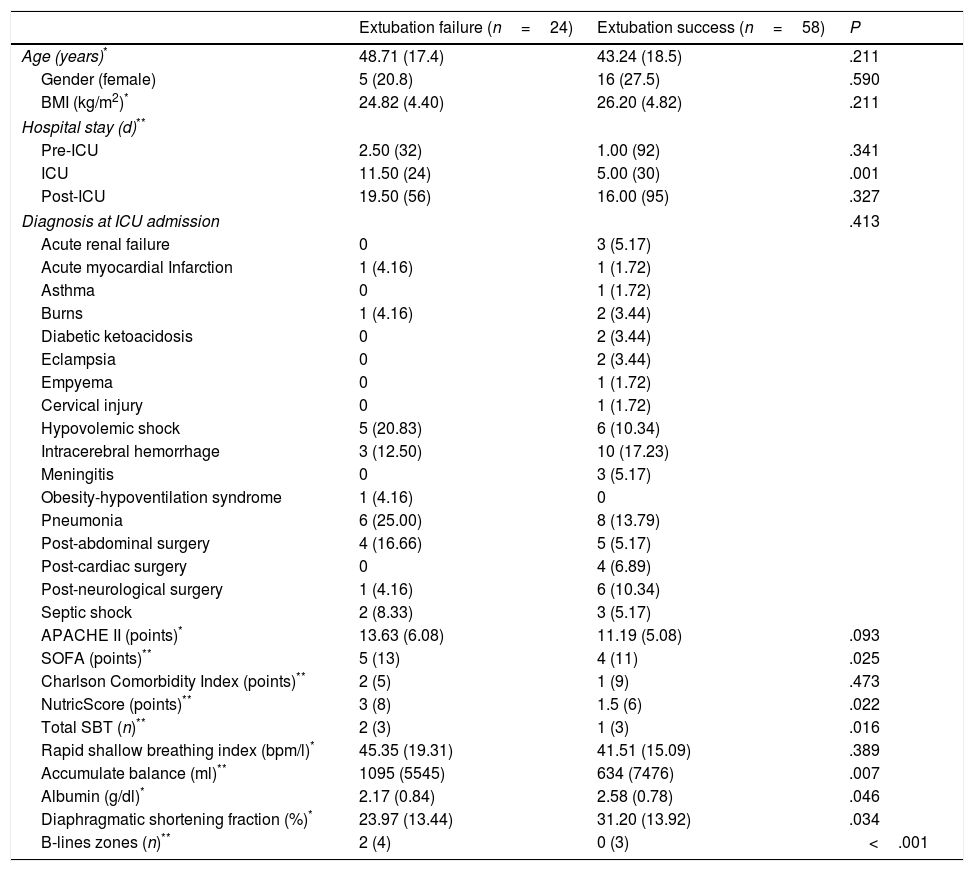
ResultsWe included 82 patients. There were no statistically significant differences between patients with success or failure to extubation respect to age, gender, body mass index, pre ICU stay, comorbidities, ICU admission diagnosis, Acute Physiology and Chronic Health disease Classification System II, or RSBI. Patients with extubation failure had statistically significant higher Sequential Organ Failure Assessment and nutritional risk score punctuation at ICU admission. Twenty-four (29.2%) failed to extubation. According to study-independent attending physician, the most frequent causes for reintubation were: respiratory insufficiency, poor secretions management, laryngospasm and bronchospasm in nine (47.5%), five (20.8%), four (16.7%) and two (8.2%) patients, respectively. Less frequent reintubation causes were: alteration of consciousness, aspiration of gastric contents, delirium, and heart failure with one (4.2%) patient each. Median time to failure was 12.2h (SD 15.52). At the moment of SBT, patients with extubation failure also had a significantly higher total liquid balance and more lung regions with B-lines; finally, albumin at the day of SBT and DSF were significantly lower in patients with extubation failure (Table 1).
General Data of Patients According to Extubation Status.
| Extubation failure (n=24) | Extubation success (n=58) | P | |
|---|---|---|---|
| Age (years)* | 48.71 (17.4) | 43.24 (18.5) | .211 |
| Gender (female) | 5 (20.8) | 16 (27.5) | .590 |
| BMI (kg/m2)* | 24.82 (4.40) | 26.20 (4.82) | .211 |
| Hospital stay (d)** | |||
| Pre-ICU | 2.50 (32) | 1.00 (92) | .341 |
| ICU | 11.50 (24) | 5.00 (30) | .001 |
| Post-ICU | 19.50 (56) | 16.00 (95) | .327 |
| Diagnosis at ICU admission | .413 | ||
| Acute renal failure | 0 | 3 (5.17) | |
| Acute myocardial Infarction | 1 (4.16) | 1 (1.72) | |
| Asthma | 0 | 1 (1.72) | |
| Burns | 1 (4.16) | 2 (3.44) | |
| Diabetic ketoacidosis | 0 | 2 (3.44) | |
| Eclampsia | 0 | 2 (3.44) | |
| Empyema | 0 | 1 (1.72) | |
| Cervical injury | 0 | 1 (1.72) | |
| Hypovolemic shock | 5 (20.83) | 6 (10.34) | |
| Intracerebral hemorrhage | 3 (12.50) | 10 (17.23) | |
| Meningitis | 0 | 3 (5.17) | |
| Obesity-hypoventilation syndrome | 1 (4.16) | 0 | |
| Pneumonia | 6 (25.00) | 8 (13.79) | |
| Post-abdominal surgery | 4 (16.66) | 5 (5.17) | |
| Post-cardiac surgery | 0 | 4 (6.89) | |
| Post-neurological surgery | 1 (4.16) | 6 (10.34) | |
| Septic shock | 2 (8.33) | 3 (5.17) | |
| APACHE II (points)* | 13.63 (6.08) | 11.19 (5.08) | .093 |
| SOFA (points)** | 5 (13) | 4 (11) | .025 |
| Charlson Comorbidity Index (points)** | 2 (5) | 1 (9) | .473 |
| NutricScore (points)** | 3 (8) | 1.5 (6) | .022 |
| Total SBT (n)** | 2 (3) | 1 (3) | .016 |
| Rapid shallow breathing index (bpm/l)* | 45.35 (19.31) | 41.51 (15.09) | .389 |
| Accumulate balance (ml)** | 1095 (5545) | 634 (7476) | .007 |
| Albumin (g/dl)* | 2.17 (0.84) | 2.58 (0.78) | .046 |
| Diaphragmatic shortening fraction (%)* | 23.97 (13.44) | 31.20 (13.92) | .034 |
| B-lines zones (n)** | 2 (4) | 0 (3) | <.001 |
Data are shown in mean and standard deviation, median and range or n and percentage, as appropriate. BMI: Body Mass Index. ICU: intensive care unit. APACHE II: Acute Physiology and Chronic Health Evaluation II. SOFA: Sequential Organ Failure Assessment.
The distribution of ultrasonographic profiles, according the BLUE-protocol,4 was: A 32 (39%), B 17 (21%), AB 19 (23%), C 11 (13%) and B’ 3 (4%) patients. Two patients with AB and two with C profiles also presented positive postero-lateral alveolar and/or pleural syndrome. A patient with a medical history of pleurodesis presented A’ profile, he was classified as A profile after ruling out pneumothorax. Failure to extubation was significantly more frequent in patients with B profile (16 vs. 3, 2, 2 and one patients with patterns A, AB, C and B’, respectively, P<.001).
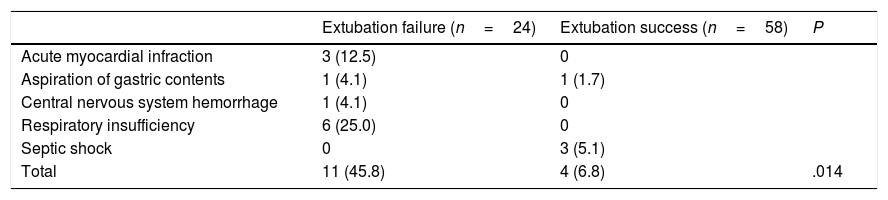
Mortality was more frequent in patients with extubation failure (45.8 vs. 6.8%, P<.001); the median for length of hospital stay was no different among the groups (19 (IQR 56) vs. 16 (IQR 95), P=.327). The survival distributions for the groups were statistically significantly different, χ2=7.31, P=.007 (Log Rank test). Table 2 shows causes of death according to extubation status at the hour 48.
Causes of Death According to Extubation Status.
| Extubation failure (n=24) | Extubation success (n=58) | P | |
|---|---|---|---|
| Acute myocardial infraction | 3 (12.5) | 0 | |
| Aspiration of gastric contents | 1 (4.1) | 1 (1.7) | |
| Central nervous system hemorrhage | 1 (4.1) | 0 | |
| Respiratory insufficiency | 6 (25.0) | 0 | |
| Septic shock | 0 | 3 (5.1) | |
| Total | 11 (45.8) | 4 (6.8) | .014 |
Data are shown as n and percentage. P obtained by Chi-square test.
Table 3 shows the area under the receiver operating characteristic curve for the most significant variables at univariate analysis, as well as the best cut point based in the combination of higher sensibility and specificity for each one. Every variable showed a better diagnostic performance than the isolated RSBI<105.
Area Under the Receiver Operator Characteristics Curve for the Prediction of Extubation Failure.
| Area | 95% CI lower bound | 95% CI upper bound | P | Youden's J | |
|---|---|---|---|---|---|
| Albumin (mg/dl) | 0.643 | 0.509 | 0.777 | .043 | <3 (87, 30) |
| Diaphragmatic shortening fraction (%) | 0.664 | 0.526 | 0.801 | .020 | <30 (62, 50) |
| Acumulate balance (ml) | 0.691 | 0.568 | 0.814 | .007 | ≥630 (70, 40) |
| B-lines zones (n) | 0.819 | 0.703 | 0.934 | <.001 | ≥1 (66, 92) |
| NutricScore (points) | 0.655 | 0.522 | 0.789 | .028 | ≥2 (54, 73) |
| Rapid Shallow Breathing Index (bpm/l) | 0.570 | 0.431 | 0.790 | .323 | ≥35 (75, 37) |
The column Youden's J shows the point with the best sensitivity and specificity (first and second numbers in parentheses, respectively) for extubation failure prediction.
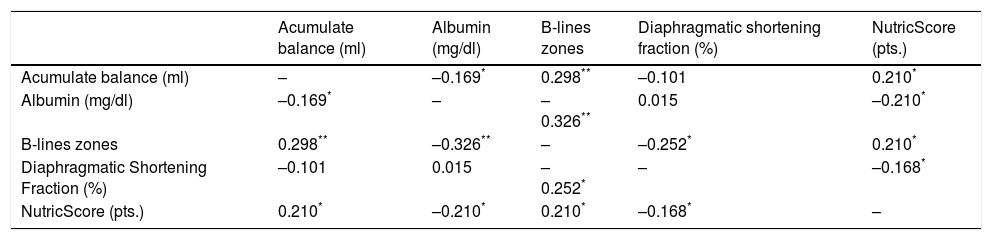
The most significative correlation was found between albumin at ICU admission and appearance of B-lines during SBT, Table 4 shows the correspondent correlation coefficients.
Correlations Between Variables Related to Extubation Failure.
| Acumulate balance (ml) | Albumin (mg/dl) | B-lines zones | Diaphragmatic shortening fraction (%) | NutricScore (pts.) | |
|---|---|---|---|---|---|
| Acumulate balance (ml) | – | –0.169* | 0.298** | –0.101 | 0.210* |
| Albumin (mg/dl) | –0.169* | – | –0.326** | 0.015 | –0.210* |
| B-lines zones | 0.298** | –0.326** | – | –0.252* | 0.210* |
| Diaphragmatic Shortening Fraction (%) | –0.101 | 0.015 | –0.252* | – | –0.168* |
| NutricScore (pts.) | 0.210* | –0.210* | 0.210* | –0.168* | – |
Correlation coefficients by Kendall's Tau method.
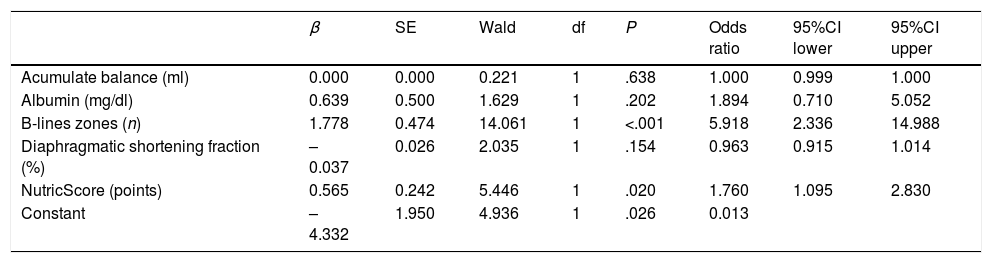
We conducted a binomial logistic regression for the accumulative balance, albumin, lung regions with B-lines, DSF and the modified nutrition risk in the critically ill (NUTRIC) Score. Based on the Box-Tidwell procedure with accepted P<.0045 derived of the Bonferroni correction, all continuous independent variables were found to be linearly related to the logit of the dependent variable. There was one studentized residual with a value of 4.739 standard deviations, which was filtered for the analysis. The logistic regression model was statistically significant, χ2(5)=41.040, P<.0005. The model explained 56% (Nagelkerke R2) of the variance in failure to extubation and correctly classified 86.4% of cases. The model sensitivity was 66.7% with a specificity of 94.7%. The positive predictive and negative value was 84, and 87%, respectively. Of the five variables analyzed, only two remained statistically significant: NUTRIC score and zones with B-lines (Table 5). Every unit increase in NUTRIC score had 1.76 (95%CI 1.09–2.83) times more odds to exhibit extubation failure. Every additional zone with B-lines increases extubation failure odds by 5.91 (2.33–14.95) times.
Binomial Logistic Regression for Variables Related to Extubation Failure in Univariate Analysis.
| β | SE | Wald | df | P | Odds ratio | 95%CI lower | 95%CI upper | |
|---|---|---|---|---|---|---|---|---|
| Acumulate balance (ml) | 0.000 | 0.000 | 0.221 | 1 | .638 | 1.000 | 0.999 | 1.000 |
| Albumin (mg/dl) | 0.639 | 0.500 | 1.629 | 1 | .202 | 1.894 | 0.710 | 5.052 |
| B-lines zones (n) | 1.778 | 0.474 | 14.061 | 1 | <.001 | 5.918 | 2.336 | 14.988 |
| Diaphragmatic shortening fraction (%) | –0.037 | 0.026 | 2.035 | 1 | .154 | 0.963 | 0.915 | 1.014 |
| NutricScore (points) | 0.565 | 0.242 | 5.446 | 1 | .020 | 1.760 | 1.095 | 2.830 |
| Constant | –4.332 | 1.950 | 4.936 | 1 | .026 | 0.013 |
Finally, weighted Kappa for concordance between ultrasound operators was 0.843 (SE 0.09) (95%CI 0.661–1.000). Observed concordance: 88.89%, expected random concordance: 58.64%.
DiscussionFailure to extubation is a frequent problem in the intensive care unit. A significant number of hospitals around the world follow the protocol established by the seminal paper of Tobin et al. However, approximately 20% of patients will present extubation failure despite a successful SBT.12 It seems clear that we must look for tools that complement the RSBI proposed since more than 20 years ago. For this purpose, we examined data from 82 medical and surgical critically ill patients. Our rate of extubation failure coincides with the reported by other authors.10 In agreement with the described in the literature, adverse clinical outcomes were more frequent among the patients with extubation failure.
At the univariate analysis, accumulate balance, serum albumin at the day of the SBT, diaphragm shortening fraction, number of performed SBT prior of the extubation, number of zones with B-lines and NUTRIC score at ICU admission were significantly related to extubation failure; however, at multivariate analysis, only the last two variables remains significant.
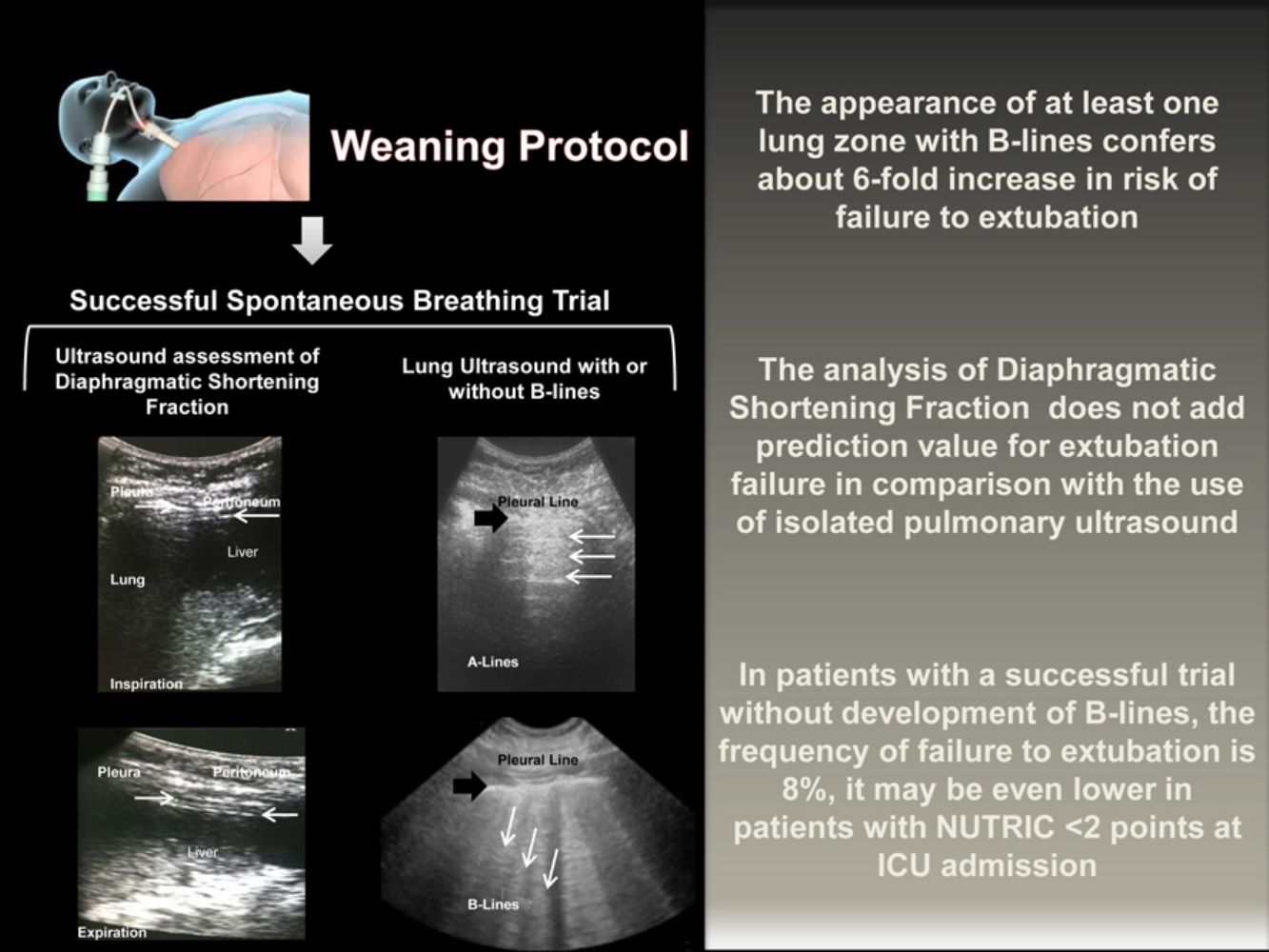
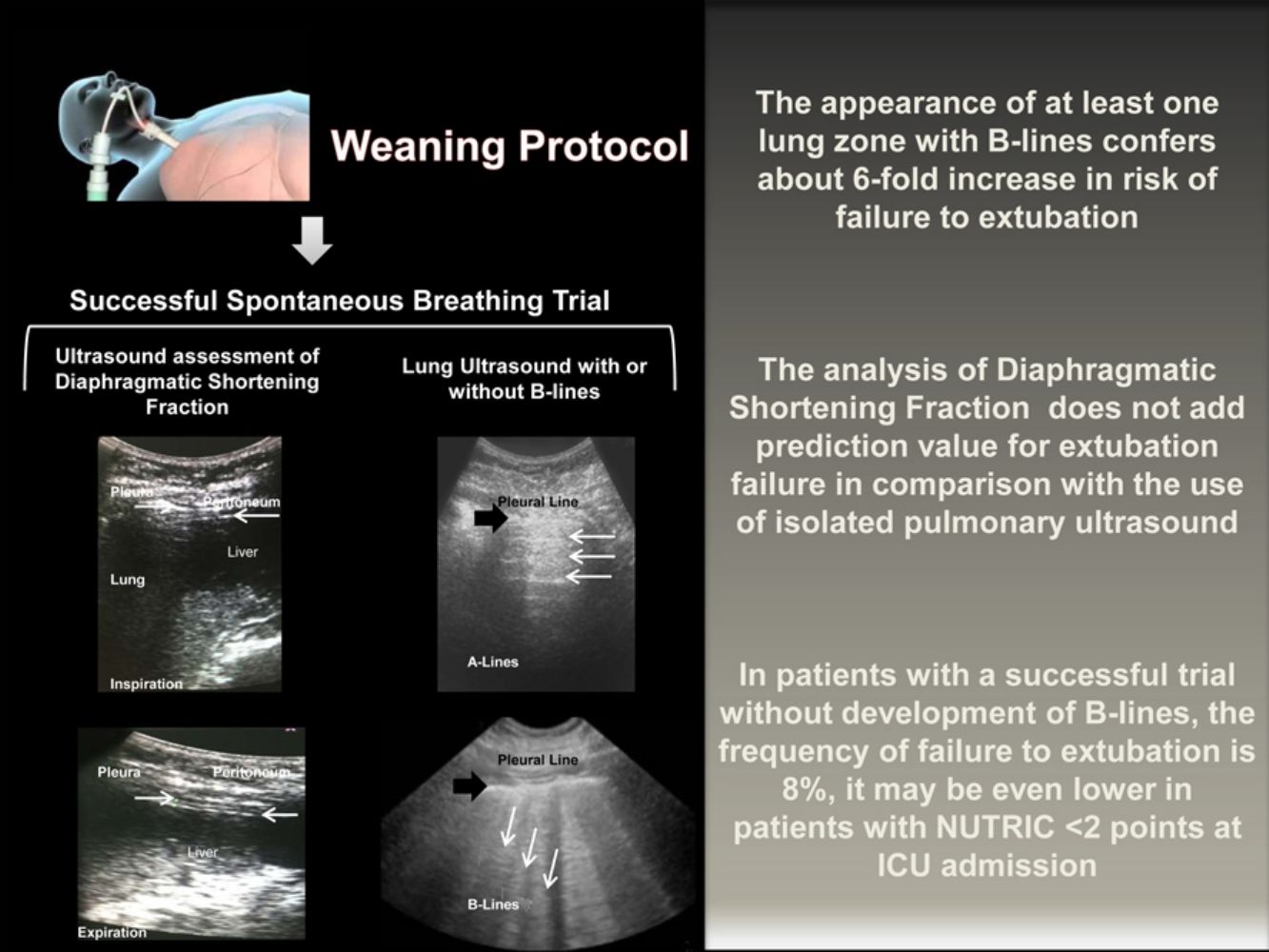
The transition from MV to spontaneous breathing is related to the rise of cardiac pre and afterload.13,14 In susceptible patients, this could lead to an increase in EVLW. The appearance of B-lines consistently correlates with the increase in EVLW measured by transpulmonary thermodilution or by pulmonary artery wedge pressure.15,16 Its development reliably predicts the declining in the PaO2/FiO2 ratio.17 On the other hand, the absence of B-lines strongly suggests EVLW less than 500ml.18 In our study, each lung zone with B-lines confers almost six-fold rise in the risk of extubation failure.
In contrast to B-lines appearance, the cumulative fluid balance at the SBT day does not remain statistically significant at the multivariate analysis. B-lines represent the final pathophysiological consequence of fluid overload. They are related to fluid balance; however, this correlation is far from being perfect (Kendall Tau 0.289); actually, serum albumin has higher correlation regard to B-lines development (Kendall Tau –0.326). The B-lines could appear in a patient with limited cardiovascular capacity at lower cumulative fluid balance in comparison to another with cardiovascular sufficiency. In this regard, the rise of serum B-type natriuretic peptide has not predicted the extubation failure.10
We consider that the identification of B-lines just before the withdrawal of the MV is relevant considering the improvement in oxygenation and respiratory mechanics related to the decrease in the EVLW. Finally, the absence of B-lines decreases the likelihood of cardiogenic edema as a cause of post-extubation respiratory insufficiency.19
The NUTRIC score predicts the occurrence of adverse outcomes among the critically ill population.20 Although this instrument does not include the variables necessary for the formal diagnosis of malnutrition, it is useful for the identification of patients who could benefit from the early implementation of nutritional therapy.20 Notwithstanding de Vries MC et al. reported the relation between high values of NUTRIC score and prolonged dependence of ventilatory support,21 to the best of our knowledge, we are the first to describe the association between NUTRIC score, even in low-risk values, and failure to extubation in patients with a successful SBT.
Diaphragmatic thickening measured in the patients of this study is concordant with the values described in the literature.8,11,22–25 We identified the DSF<30% as the best cut-point for the prediction of extubation failure. The above is in line with the value proposed by DiNino et al.11 and is slightly different from the values of 20 and 36% proposed by Blumhof et al. and Ferrari et al., respectively.8,24
Even though the sensibility, specificity, and under the curve reported by these authors are higher than the values reported in this paper, there are several differences in the protocol design between the studies. In contrast to DiNino et al., we performed all the SBT in a single fashion way (30min of support pressure and continuous positive airway pressure of zero cmH2O). Besides, we evaluated the DSF at the end of the successful SBT, and we proceeded immediately with the extubation. Otherwise, primary objectives of Blumhof et al. and Ferrari et al. were not directly related to the measure of the extubation failure risk. The first one predicted the medical-team-decision to perform the extubation in the next 48h since diaphragmatic evaluation and Ferrari et al. analyzed the probability of SBT success exclusively in patients with prolonged weaning.
We consider that lack of statistical significance of the DSF for the prediction of extubation failure in the multivariate analysis could be due to the studied population. Even though the diaphragmatic dysfunction is widespread among mechanically ventilated patients,26,27 diaphragmatic performance considerably improves throughout the ICU stay.23 The use of small driving pressures and ventilation modes that allow spontaneous breathing, like the ones we routinely use in our ICU, were related to the recuperation of the DSF.23 Despite we do not have baseline DSF measurements, it is possible that by the time of SBT, most patients had had a diaphragmatic dysfunction recovery, in case that it was present at ICU admission. It may be possible that the successful SBT selects a majority of subjects without diaphragmatic dysfunction as a cause of potential extubation failure.
All the parameters studied showed a greater area under the curve than the RSBI<105bpm/l. We consider that the cut-off proposed by Tobin et al. should not be modified; although in our analysis the point of highest sensitivity and specificity was 35bpm/l, this cut-point is impractical because a large number of patients would remain in MV in virtue to decrease the frequency of failure to extubation.
The limitations of our study are: (1) only 10 (12.19%) patients underwent tracheostomy, and just 6 (7.31%) required ventilatory support for more than 14 days. Accordingly, our results may not apply to patients with prolonged ventilatory support. (2) We do not perform the measure of inspiratory occlusion pressure at the 0.1s; therefore, although all of our patients performed the SBT out of sedation, we cannot exclude residual drug effect as a cause of a decrease in the motor diaphragm outflow.
We consider that the non-inclusion of echocardiography in our protocol is not a limitation for the following reasons: (1) echocardiogram alone does not predict failure to extubation.10 (2) Although the measurement of the ejection fraction of the left ventricle can be performed with relative ease by non-cardiologists, pulmonary edema can be present with preserved left ventricular ejection fraction in up to 50% of patients.28 (3) The adequate assessment of diastolic function requires formal training that may not be part of most intensive therapy physicians’ skills. (4) The completion of a comprehensive echocardiogram by an experienced cardiologist should be considered in all cases of suspected cardiac compromise (before SBT), the appearance of B lines (during SBT) or failure to extubation (after a successful SBT).
The strengths of this study consists of an adequate calculation of the sample size, as well as the performance of multivariate analysis and the inclusion of medical and surgical patients with a well-established MV weaning protocol. Finally, the concordance of the ultrasound measurements was excellent, approaching 90%, which adds certainty to our results.
In conclusion, the routine realization of pulmonary ultrasound at the end of the SBT might add safety to extubation. If the patient complies a successful SBT without appearance of B-lines, the frequency of failure to extubation is around 8%, and may even be lower in patients with a NUTRIC score<2 points at the ICU admission. Given the high positive and negative predictive values of the pulmonary ultrasound, its complementation with diaphragmatic ultrasound it is not necessary. It may be appropriate to delay the extubation of patients who develop B-lines in the course of a successful SBT to adequately treat venocapillary pulmonary hypertension, however, prospective clinical trials should evaluate this strategy.
Conflict of InterestThe authors declare no conflict of interests.
FundingThe equipment used to conduct the present research was provided by the Department of Pulmonary and Critical Care Medicine of the “Dr. José E. González” University Hospital. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
AuthorshipJEG designed the study and had full access to all the data in the study, takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects, and assume full responsibility for the integrity of the submission as a whole, from inception to published article. CPR, EJR, RM, RC and JAS contributed substantially to the acquisition, analysis and interpretation of data, and the writing of the manuscript.
To Erick W. Renpenning-Carrazco, MD for the coordination and realization of ultrasound studies; to Héctor E. Cedillo-Huerta, MD for data acquisition, and to Roxana Saldaña-Vázquez, MD for her assistance in the review of the paper.