Patients with pre-existing respiratory diseases in the setting of COVID-19 may have a greater risk of severe complications and even death.
MethodsA retrospective, multicenter, cohort study with 5847 COVID-19 patients admitted to hospitals. Patients were separated in two groups, with/without previous lung disease. Evaluation of factors associated with survival and secondary composite end-point such as ICU admission and respiratory support, were explored.
Results1,271 patients (22%) had a previous lung disease, mostly COPD. All-cause mortality occurred in 376 patients with lung disease (29.5%) and in 819 patients without (17.9%) (p<0.001). Kaplan–Meier curves showed that patients with lung diseases had a worse 30-day survival (HR=1.78; 95%C.I. 1.58–2.01; p<0.001) and COPD had almost 40% mortality. Multivariable Cox regression showed that prior lung disease remained a risk factor for mortality (HR, 1.21; 95%C.I. 1.02–1.44; p=0.02). Variables independently associated with all-cause mortality risk in patients with lung diseases were oxygen saturation less than 92% on admission (HR, 4.35; 95% CI 3.08–6.15) and elevated D-dimer (HR, 1.84; 95% CI 1.27–2.67). Age younger than 60 years (HR 0.37; 95% CI 0.21–0.65) was associated with decreased risk of death.
ConclusionsPrevious lung disease is a risk factor for mortality in patients with COVID-19. Older age, male gender, home oxygen therapy, and respiratory failure on admission were associated with an increased mortality. Efforts must be done to identify respiratory patients to set measures to improve their clinical outcomes.
Los pacientes con enfermedades respiratorias preexistentes pueden tener en el contexto de la covid-19 un mayor riesgo de complicaciones graves e incluso de muerte.
MétodosEstudio de cohortes multicéntrico y retrospectivo de 5.847 pacientes con covid-19 ingresados en hospitales. Los pacientes se separaron en 2 grupos, sin y con enfermedad pulmonar previa. Se evaluaron factores asociados con la supervivencia y criterios combinados de valoración secundarios, como el ingreso en la UCI y la necesidad de asistencia respiratoria.
ResultadosMil doscientos setenta y un (1.271) pacientes (22%) tenían una enfermedad pulmonar previa, principalmente EPOC. La mortalidad por todas las causas ocurrió en 376 pacientes con enfermedad pulmonar (29,5%) y en 819 pacientes sin enfermedad pulmonar (17,9%; p<0,001). Las curvas de Kaplan-Meier mostraron que los pacientes con enfermedades pulmonares tenían una peor supervivencia a los 30 días (HR: 1,78; IC del 95%: 1,58-2,01; p<0,001) y la EPOC tenía una mortalidad de casi el 40%. La regresión de Cox multivariante mostró que la enfermedad pulmonar previa seguía siendo un factor de riesgo de mortalidad (HR: 1,21; IC del 95%: 1,02-1,44; p=0,02). Las variables asociadas de forma independiente con el riesgo de muerte por todas las causas en pacientes con enfermedades pulmonares fueron la saturación de oxígeno inferior al 92% al ingreso (HR: 4,35; IC del 95%: 3,08-6,15) y el dímero D elevado (HR: 1,84; IC del 95%: 1,27-2,67). La edad menor de 60 años (HR: 0,37; IC del 95%: 0,21-0,65) se asoció con una disminución del riesgo de muerte.
ConclusionesLa enfermedad pulmonar previa es un factor de riesgo de muerte en pacientes con covid-19. La edad avanzada, el sexo masculino, la oxigenoterapia domiciliaria y la insuficiencia respiratoria al ingreso se asociaron con un aumento de la mortalidad. Se deben realizar esfuerzos para identificar a los pacientes respiratorios y establecer medidas para mejorar sus resultados clínicos.
On December 31, 2019, a cluster of pneumonia of unknown cause was reported in Wuhan, Hubei province of China, and ten days later a novel coronavirus (SARS-CoV-2) was identified.1 Although control measures were applied locally, the spread of infection increased and the World Health Organization (WHO) declared a global health emergency.2
Previous studies3,4 have identified several clinical features of patients with this new disease named as COVID-19. The majority of patients seem to have mild disease, but about 20% will progress to severe disease, including pneumonia, respiratory failure, need of mechanical ventilation and even death.
Several studies1,5–7 showed that patients with comorbidity, infected with SARS-CoV-2, might have a poorer prognosis. Cardiovascular disease, malignancy, hypertension, diabetes, cerebrovascular disease and renal disease8 were risk factors in COVID-19 patients.9 Age is another risk factor and older patients tend to have more complications.10,11
Prevalence of lung diseases has grown globally and nearly 545 million individuals currently live with a chronic respiratory condition, representing 7.4% of the world's population.12 Since the emergence of COVID-19 several studies3,4,7 have highlighted a low frequency of respiratory patients hospitalized for infection by SARS-CoV-2. However, it appears that respiratory patients are at greater risk of severe complications and even death.13–15
The aim of our study is to analyze the prevalence, clinical profile and complications of COVID-19 patients admitted to hospital with prior lung disease in a multicenter international cohort.
MethodsStudy design and populationThe Health Outcome Predictive Evaluation for COVID-19 (HOPE-COVID-19) is a multicenter, international, real-life registry, with study design as a retrospective clinical cohort aimed to identify determinants of infection and prognosis of COVID-19 patients (clinicaltrials.gov NCT04334291). All adult patients admitted to hospital for COVID-19 or those deceased were suitable for the study. There were no exclusion criteria, except for patients or families’ explicit refusal to participate. Patients from 41 centers in 30 cities and 6 countries (Canada, China, Cuba, Ecuador, Germany, Italy and Spain) were included. Detailed information about participating countries and hospitals, protocol and definitions are reported on Supplement (e-Appendix 1, e-Tables 1 and 2). In this analysis clinical outcomes are presented for patients admitted since March 23, 2020 who completed their hospital course as of May 5, 2020 (discharged alive or dead).
Data sourceDemographic, clinical, and outcome data were extracted from electronic medical records in all participating centers. Confidentiality was guaranteed by typing all patient information anonymously and stored in a password-protected secure online database (www.hopeprojectmd.com).
Confirmed COVID-19 cases were those with a positive nasal and pharyngeal swab sample obtained at admission using real time reverse transcriptase-polymerase chain reaction (RT-PCR) as per WHO recommendations. Data included comorbidities (hypertension, diabetes, dyslipidemia, obesity, smoking, lung, heart, cerebrovascular, renal, liver and connective tissue disease, cancer, dementia, etc.); emergency room assessment variables, clinical assessments during hospitalization (radiology, laboratory findings, clinical signs and symptoms, severity as use of ventilatory support or admission to intensive care unit [ICU], etc.); and discharge status. All procedures and treatments were applied by the medical team in each center, following clinical guidelines and protocols.
Study outcomesWe selected those patients that had a prior diagnosis of lung disease, namely chronic obstructive pulmonary disease (COPD), asthma, interstitial lung disease (ILD, mainly pulmonary fibrosis) and other (sleep apnea, bronchiectasis, tuberculosis sequela, etc.).
Additionally, a pragmatic decision was adopted so we also included patients who received inhalers (steroids or beta-agonists) as regular medication or supplemental oxygen at home, independently of being registered without previous lung disease.
We hypothesized that pre-existing lung disease in patients admitted with COVID-19 lead to worse outcome, so our primary end-point was 30 day-mortality and associated factors. Secondary composite end-points were need for ICU admission and need of mechanical ventilation, non-invasive mechanical ventilation and high flow nasal cannula oxygen. Complications as pneumonia, sepsis, hemoptysis, embolic events and heart failure were also investigated. The protective role of previous medications or in-hospital treatments was also analyzed.
Ethical issuesThe study was classified by Spanish Drug Agency authorities (AEMPS classification: EPA-0D) and approved by Ethics Research Committee of the coordinating center (Hospital Clinico San Carlos, Madrid, Spain) (20/241-E). Some local committees accepted this approval but other required their own approval, such as centers in Galicia (Spain), in Canada, in Cuba, and in Ecuador. Written informed consent was waived owing to the severity of the situation and the use of deidentified retrospective data. However, verbal authorization from either patients or caregivers was required.
Statistical analysisWe followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)16 guidelines for reporting observational studies. Continuous variables were expressed as median (IQR) or as mean and standard deviation, and either Student's t test or the Mann–Whitney U test were used to test the significance of comparisons. Categorical variables were expressed as number (%) and compared by χ2 test or Fisher's exact test between groups (patients with previous lung disease or not). Survival analysis of patients with or without prior lung disease and type of lung disease categories (COPD, asthma, ILD, other) was performed with Kaplan–Meier using Log-rank test. Cox Regression was used in the multivariable approach of survival. Variables included were: Age (continuous), gender, comorbidities (hypertension, diabetes), smoking history, home oxygen therapy, previous inhaled steroids, oxygen saturation below 92% at admission, elevated D-dimer (≥0.5mg/l), lymphocyte count <1500 per mm3, treatments (intravenous steroids, chloroquine/hydroxychloroquine [CQ/HCQ], tocilizumab), and abnormalities on chest radiograph. Model selection was performed according to backward stepwise approach. The removal of a variable from the model was based on the significance of the Wald statistic (p>0.1). No imputation was made for missing data. Statistical analysis was generated using SPPS statistics v24.0 (SPSS Inc., Chicago, IL, USA) and R (version 3.6.2) for Kaplan–Meier curves.
A two-sided α of less than 0.05 was considered statistically significant.
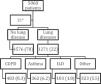
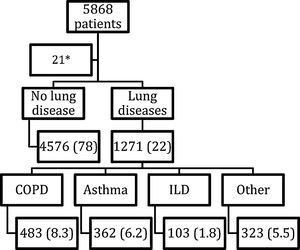
ResultsDescription of the cohortAs of May 5, 2020, a total of 5,868 patients were included in the registry HOPE-COVID-19. After the withdrawal of 21 younger than 18 years individuals, 5847 patients were considered (Fig. 1). We divided them in two groups: 4756 had no prior lung disease (78%), and 1271 had any lung disease (22%). Those were mostly COPD patients, 483 (8.3% of the registry), followed by patients with asthma, 362 (6.2%), ILD, 103 (1.8%), and other respiratory, 323 (5.5%).
Study flow chart.14 Data are expressed as absolute numbers and percentage referred to the whole group. COPD: Chronic Obstructive Respiratory Disease. ILD: Interstitial Lung Disease. * 21 patients withdrew due to age less tan 18 years.
Patients with lung diseases were older (70.8±14.7 vs. 63.6±16.8; p<0.001), mostly male (64.2% vs. 57.2%; p<0.001) and as expected, they had been exposed to tobacco (current smokers or ex-smokers) more than the group with no lung disease (43.8% vs. 18.1%; p<0.001). Comorbidities as diabetes, hypertension, obesity, cardiovascular diseases, chronic renal disease, cancer, frailty and previous immunosuppressive treatments were more prevalent in the lung diseases group (all p<0.001). Demographics, clinical characteristics, radiologic and laboratory findings, complications and clinical outcomes of the patients are shown in Table 1.
Clinical characteristics of the study patients according to the presence of lung disease. Age is expressed by mean±SD. CQ/HCQ: chloroquine/hydroxychloroquine. Composite end-point: admission to an ICU and the need of respiratory support (mechanical ventilation, non-invasive mechanical ventilation and high flow nasal cannula oxygen).
| All patients | No lung disease | Any lung disease | p value | |
|---|---|---|---|---|
| (N=5847) | (N=4576) | (N=1271) | ||
| Age | 65.1±16.6 | 63.6±16.8 | 70.8±14.7 | p<0.001 |
| Gender (female) ______no. (%) | 2415 (41.3) | 1959 (42.8) | 456 (35.8) | p<0.001 |
| Race _____no./total (%) | ||||
| Black | 46/5847 (0.8) | 41/4753(0.9) | 5/1274 (0.4) | p>0.05 |
| Caucasian | 4880/5847(83.5) | 3725/4753(81.5) | 1155/1274 (90.7) | p<0.001 |
| Latin | 748/5847 (2.3) | 652/4753(14.3) | 96/1274 (7.5) | p>0.05 |
| Oriental | 132/5847 (2.3) | 126/4753 (2.8) | 6/1274 (0.5) | p>0.05 |
| Other | 41/5847 (0.7) | 29/4753 (0.6) | 12/1274 (0.9) | p>0.05 |
| Smoking _____no. (%) | ||||
| Never smoked | 3963 (76.2) | 3311 (81.9) | 652 (56.2) | p<0.001 |
| Former smoker | 934 (17.9) | 525 (13.0) | 409 (35.2) | |
| Current smoker | 307 (5.9) | 207 (5.1) | 100 (8.6) | |
| Comorbidities _____ no. (%) | ||||
| Obesity | 1036 (22.4) | 686 (19.0) | 350 (34.4) | p<0.001 |
| Diabetes | 1093 (18.7) | 761 (16.6) | 332 (26.1) | p<0.001 |
| Hypertension | 2866 (49.2) | 2062 (45.3) | 804 (63.5) | p<0.001 |
| Heart disease | 1335 (23) | 883 (19.5) | 452 (35.8) | p<0.001 |
| Chronic renal disease | 383 (6.6) | 240 (5.3) | 143 (11.2) | p<0.001 |
| Cancer | 773 (13.5) | 525 (11.7) | 248 (19.9) | p<0.001 |
| Frailty | 767 (13.3) | 509 (11.3) | 258 (20.6) | p<0.001 |
| Previous immunosuppressive treatments | 416 (7.7) | 290 (6.6) | 126 (12.4) | p<0.001 |
| Radiologic findings _____no. (%) | ||||
| Local patchy shadowing | 1036 (19.5) | 809 (19.5) | 227 (19.3) | p=0.91 |
| Bilateral patchy shadowing | 3591 (67.4) | 2802 (67.5) | 789 (67.2) | |
| Laboratory findings ____no. (%) | ||||
| O2 saturation<92% | 2000 (35.4) | 1418 (32.1) | 582 (46.9) | p<0.001 |
| D-dimer≥0.5mg/dl | 3199 (64.7) | 2459 (63.2) | 740 (70.1) | p<0.001 |
| Procalcitonin≥0.5ng/dl | 903 (21.8) | 679 (20.9) | 224 (25.3) | p=0.03 |
| C-reactive protein≥10mg/liter | 5024 (89.2) | 3892 (88.4) | 1132 (92.1) | p<0.001 |
| Ferritin≥250ng/dl | 1795 (59.6) | 1421 (59.6) | 374 (59.6) | p=1.0 |
| Lactate dehydrogenase≥250U/liter | 3726 (72.2) | 2883 (71.4) | 843 (75.0) | p=0.09 |
| Creatinine≥133μmol/liter | 809 (14.4) | 581 (13.2) | 228 (18.6) | p<0.001 |
| White-cell count<4000 per mm3 | 835 (14.7) | 665 (15) | 170 (13.7) | p=0.239 |
| Lymphocyte count<1500 per mm3 | 4304 (77.4) | 3333 (76.8) | 971 (79.5) | p=0.048 |
| Platelet count<150,000 per mm3 | 1430 (25.3) | 1085 (24.6) | 345 (27.8) | p=0.024 |
| Hemoglobin<12g/dl | 1460 (25.9) | 1084 (24.6) | 376 (30.4) | p<0.001 |
| Complications _____no. (%) | ||||
| Acute renal failure | 928 (16.2) | 663 (14.8) | 265 (21.3) | p<0.001 |
| Septic shock | 625 (11) | 464 (10.5) | 161 (13.1) | p=0.06 |
| Acute inflammatory syndrome | 1105 (19.6) | 845 (19.1) | 260 (21.2) | p=0.06 |
| Embolic events | 120 (2.1) | 85 (1.9) | 35 (2.9) | p=0.03 |
| ICU admission | 529 (9) | 408 (8.9) | 121 (9.5) | p=0.28 |
| Treatments _____no. (%) | ||||
| Supplemental O2 | 4076 (71.1) | 3206 (68.9) | 870 (80.8) | p<0.001 |
| High flow O2 nasal can. | 1127 (19.9) | 859 (19.4) | 268 (21.7) | p=0.04 |
| Noninvasive mech. vent | 783 (13.7) | 591 (13.3) | 192 (15.5) | p=0.02 |
| Mechanical ventilation | 416 (7.4) | 323 (7.3) | 93 (7.5) | p=0.41 |
| Use of steroids | 1554 (27.5) | 1117 (25.2) | 437 (35.4) | p<0.001 |
| Use of CQ/HCQ | 4854 (84.6) | 3784 (84.4) | 1070 (85.4) | p=0.20 |
| Use of antiviral drugs | 3454 (60.4) | 2750 (61.5) | 704 (56.4) | p=0.001 |
| Use of interferon | 743 (13.2) | 596 (13.5) | 147 (12.0) | p=0.09 |
| Use of tocilizumab | 475 (8.4) | 366 (8.3) | 109 (8.9) | p=0.25 |
| Length of stay in days (mean, SD) | ||||
| Hospital general ward | 9.78 (9.78) | 9.79 (9.78) | 9.55 (9.25) | p=0.502 |
| Intensive care unit | 11.01 (7.43) | 11.01 (9.63) | 10.19 (7.43) | p=0.484 |
| Death _____no. (%) | 1195 (20.4) | 819 (17.9) | 376 (29.5) | p<0.001 |
| Composite end-point_____no.(%) | 1707 (29.2) | 1306 (28.5) | 401 (31.5) | p=0.02 |
Radiologic findings on admission revealed no difference between both groups of patients, and abnormalities on chest X ray were present in more than 85% of patients (local or bilateral patchy shadowing). Patients with lung disease had lower oxygen saturation at admission (47% vs. 32% had O2 saturation below 92%; p<0.001), but higher D-dimer, procalcitonin, C-reactive protein and creatinine and lower number of lymphocytes, platelets and hemoglobin compared with those with no previous lung disease. However, no differences were found in lactate dehydrogenase, white cell count and ferritin (Table 1).
Complications and treatmentsPatients with lung diseases had during hospitalization more often acute renal failure and embolic events. Compared with them, patients without lung disease had similar numbers of pneumonia, septic shock and abnormal inflammatory response syndrome.
Regarding respiratory support, more patients with lung disease had noninvasive mechanical ventilation and received conventional supplemental oxygen or through a high flow nasal cannula (HFNC) but no differences were found between groups in the number of patients who were intubated and had mechanical ventilation (7.5% vs. 7.3%).
Most patients (75.7%) received intravenous antibiotics, and systemic steroids were given more often to previous lung disease patients (35.4% vs. 25.2%; p<0.001), but antiviral therapies were administered in more patients with no lung disease (61.5 vs. 56.4; p<0.001). Au contraire, no differences were found in several therapies commonly used for COVID-19 infection such as interferon, CQ/HCQ or tocilizumab (Table 1).
Clinical outcomes and survival analysisLength of hospitalization was 9.78 days in general wards and 11.01 days in intensive care units, without differences between both groups of patients (Table 1). All cause mortality, occurred in 376 out of 1274 patients with lung disease (29.5%) and in 819 patients without lung disease (17.9%) (p<0.001). Regarding secondary composite end-point (admission to ICU and the need of respiratory support) event, it occurred in more patients with previous lung disease (401 [31.5%] vs. 1306 [28.5%]; p=0.02) (Table 1).
Cause of death was collected in 1174 patients and the majority of them (84%) died due to respiratory causes (984 patients). No differences were found between groups (previous lung disease versus none).
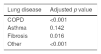
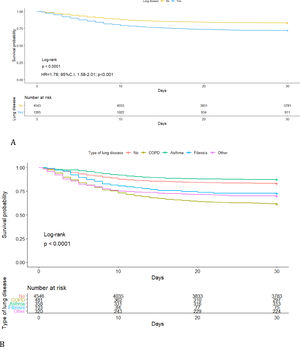
Kaplan–Meier curves showed that patients with lung diseases had a worse 30-day survival than those without preexisting lung disease (log-rank p<0.001) (HR=1.78; 95%C.I. 1.58–2.01; p<0.001) (Fig. 2A). When we compared within each lung disease, COPD had the worse survival (Fig. 2B) with almost 40% mortality at 30 days (Supplement, e-Table 3). On the other hand, no differences in survival were found between patients with asthma and patients with no lung disease (p=0.142) (Table 2). Several analyses were performed to compare patients with prior lung diseases with or without extra-pulmonary comorbidities versus those with no comorbidities and survival probability was lower in patients with previous pulmonary disease and other extra-pulmonary comorbidities (Supplement, e-Table 4, e-Figure 1).
Post hoc Cox proportional hazard regression for survival between different lung diseases versus none. p value adjustment according to Benjamini.33
| Lung disease | Adjusted p value |
|---|---|
| COPD | <0.001 |
| Asthma | 0.142 |
| Fibrosis | 0.016 |
| Other | <0.001 |
Multivariable Cox regression was also performed; resulting in prior lung disease remained a prognostic factor for survival. Patients with lung disease had a 21% greater mortality risk (HR=1.21; 95%C.I. 1.02–1.44; p=0.02) compared to those without previous lung disease (Table 3).
Multivariable analysis with Cox regression for all COVID-19 patients included in the registry (backward-forward stepwise selection: AIC).a
| Characteristics | Hazard ratio | 95% C.I. | p value |
|---|---|---|---|
| Prior lung disease | 1.21 | 1.02–1.44 | 0.02 |
| Gender | 1.24 | 1.05–1.46 | 0.01 |
| Hypertension | 1.76 | 1.46–2.13 | <0.001 |
| Diabetes | 1.29 | 1.08–1.54 | <0.001 |
| SaO2<92% | 4.20 | 3.50–5.04 | <0.001 |
| Elevated D-dimer | 1.73 | 1.39–2.15 | <0.001 |
| Lymphocyte<1500 | 1.41 | 1.10–1.79 | <0.001 |
| Unilateral chest X-ray abnormality | 0.96 | 0.67–1.38 | 0.84 |
| Bilateral chest X-ray abnormality | 1.28 | 0.94–1.74 | 0.11 |
| Age | 0.33 | 0.25–0.43 | <0.001 |
Variables included in the model: Age (categorized ≤60 vs. ref. >60 years), gender (male), diabetes, hypertension, smoking history, previous inhaled steroids, oxygen saturation below 92% at admission, elevated D-dimer (≥0.5mg/l) at admission, lymphocyte count<1500 per mm3 at admission, and abnormalities on chest radiograph (unilateral or bilateral vs. none).
Finally, results from Cox regression analyses in patients with prior lung diseases are presented in Table 4. Here, gender (male) (HR 1.32; 95%CI 0.98–1.78), hypertension (HR 1.33; 95%CI 0.96–1.84), use of home supplemental oxygen (HR 1.37; 95%CI 0.98–1.92) were not statistically associated with all-cause mortality in patients with lung disease. However, oxygen saturation less than 92% at admission, showed a four-fold increase risk in mortality (HR 4.35; 95%CI 3.08–6.15); elevated D-dimer (HR 1.84; 95%CI 1.27–2.67), and treatment with systemic steroids (HR 1.37; 95%CI 1.04–1.80) all emerged as risk factors. Au contraire, treatment with CQ/HCQ (HR 0.53; 95% CI 0.37–0.75) and age younger than 60 years (HR 0.37; 95% CI 0.21–0.65) were associated with significantly better survival.
Multivariable analysis with Cox regression for patients with lung disease (backward-forward stepwise selection: Wald).a
| Characteristic | Hazard ratio | 95% C.I. | p value |
|---|---|---|---|
| Gender | 1.32 | 0.98–1.78 | 0.067 |
| Hypertension | 1.33 | 0.96–1.84 | 0.090 |
| Home oxygen therapy | 1.37 | 0.98–1.92 | 0.065 |
| SaO2<92% | 4.35 | 3.08–6.15 | <0.001 |
| Elevated D-dimer | 1.84 | 1.27–2.67 | 0.001 |
| Use of intravenous steroids | 1.37 | 1.04–1.80 | 0.025 |
| Use of CQ/HCQ | 0.53 | 0.37–0.75 | <0.001 |
| Age | 0.37 | 0.21–0.65 | 0.001 |
aVariables included in the model: Age (categorized≤60 vs. ref.>60 years), sex (male), comorbidities (hypertension, diabetes), smoking history, home oxygen therapy, previous inhaled steroids, oxygen saturation below 92% at admission, elevated D-dimer (≥0.5mg/liter), lymphocyte count<1500 per mm3, treatments (intravenous steroids, CQ/HCQ, tocilizumab), and abnormalities on chest radiograph. Omnibus test of model coefficients (−2 log likehood: 2761.96).
This is a multinational, retrospective clinical cohort study and to date is one of the largest research studies exploring the effect of previous lung disorders in COVID-19 patients admitted to a hospital. In our registry, 1271 patients (22%) had a respiratory disease upon admission, and having a prior diagnosis of lung disease is a risk factor for a worse prognosis and even death.
Early studies3–5 showed that the majority of infected patients would have a mild disease, however some of them will develop a severe disease, and comorbidities play a major, modifying role. Surprisingly, patients with chronic respiratory disease were underrepresented as compared with other comorbidities as cerebrovascular or cardiovascular disease. Huang3 presented a series of 41 patients with 13 of them admitted to ICU. One third had any comorbidity, such as diabetes (20%), hypertension (15%) and only 1 patient had a previous lung disease.
Similar prevalence were extracted from Guan,4 where only 12 patients (1%) had COPD, but half of them were admitted to ICU, mechanically ventilated or died. In a series of 138 patients, Wang5 described a 2.9% prevalence of COPD in their study but again, 8.3% of patients admitted to ICU had previous lung diseases. In a larger study, Guan7 analyzed the impact of comorbidities in 1590 patients. They found that only 1.5% had COPD, with no data about other respiratory disorders such as asthma or ILD. Unexpectedly only 7% of their patients were exposed to tobacco (current or former). Mortality rate of COPD patients was 25% with an HR of 2.68, after adjusting for age and sex, to reach any of the composite end points (ICU admission, mechanical ventilation or dead). In another study11 from China, about risk factors associated with acute respiratory distress syndrome, only 5 patients (2.5%) had chronic lung disease.
These figures about prevalence of previous respiratory diseases in COVID-19 patients admitted to hospital change when we analyze data from elderly patients or from western countries. In a study17 of 339 patients older than 60 years, there were 6.2% COPD patients, with a HR of 2.24 for complications. Fatal cases were almost 20% and 17% of them had COPD. A study18 from the New York City area showed that the most common comorbidities were hypertension, diabetes and obesity, but they also collected data in chronic respiratory diseases and 9% of patients had asthma, 4.5% had COPD and 2.9% had obstructive sleep apnea.
Angiotensin converting enzyme II (ACE2) was described as a cell entry receptor for the coronavirus responsible of SARS.19 With the outbreak of COVID-19, researchers20 confirmed that SARS-CoV-2 uses the same ACE2 receptor. Analysis21 of resected lung tissue specimens obtained from smokers with COPD, “healthy” smokers and non-smokers showed an increase of ACE2 gene expression in the epithelial cells of COPD vs. control and of current smokers vs. never smokers. Moreover, there was a significant inverse relationship between ACE2 gene expression and FEV1%. This up-regulation may predispose COPD patients or smokers to increased risk of COVID-19 disease.
Coronaviruses have been related to acute exacerbations of COPD. In a cohort22 of 200 patients from the United States and Europe, bacterial taxa and virus were studied in stable state and during an exacerbation event. Coronavirus was detected in 5% of sputum samples and it was associated with an acute event and also with frequent exacerbations. A retrospective analysis23 in COPD patients showed that coronavirus ranked as the third cause, after influenza A, and respiratory syncytial virus, for emergency department visits and hospitalizations.
Differences in prevalence of respiratory diseases in patients admitted to a hospital for COVID-19 may have several explanations. First of all, age. Our registry is based mostly of patients from Italy and Spain, where COVID-19 patients were older (mean age 65 years) than previously published series from elsewhere. This may represent European population aging, and COPD and ILD prevalence increases with age. Second, underdiagnosis of all chronic respiratory diseases is a universal feature.12 Data from epidemiological studies24 show that COPD patients are commonly underdiagnosed due to physician unawareness, spirometry unavailability, confounding of symptoms with tobacco smokers and heart conditions, among other. Third, our registry divided patients in several categories of lung disease, which may increase attention in declaring it by the local researchers. Fourth, it may be related to a protective effect of COPD treatments, as inhaled steroids. A study from Japan25 showed a reduction of coronavirus (not SARS-CoV-2) replication and cytokine production in cultures of cells from nose and trachea after treatment with budesonide. We collected information about previous treatments in our series of patients, but no effect was found with neither the use of inhaled beta-agonists nor inhaled steroids. A recent systematic review of inhaled steroids in COVID-19 found zero trials to meta-analyse,26 no publications were identified as having data on prior ICS use in patients with SARS, MERS or COVID-19 infection. No data were available for either a qualitative or narrative answer to the review question.
Complications and mortality are increased in COVID-19 patients with comorbidities as chronic respiratory disorders. A study15 in patients admitted to intensive care units in Italy, almost three fourth of them with COPD died and it was an independent risk factor associated with mortality (HR, 1.68; 95% CI, 1.28–2.19). In our study, 30% of patients with previous lung disease died, compared with 18% of patients without respiratory diseases. Mortality in the sub-group of COPD patients was even higher, 40% of them died. They represent 16% of all declared deaths in our registry.
Contrary, patients with asthma appear not to have an increased risk of mortality. There are contradictory results regarding this issue, as a recent study27 showed that COVID-19 patients with asthma had a higher mortality rate, whilst a meta-analysis28 indicated that asthma as a comorbidity may not increase mortality.
A number of meta-analyses have explored the risk of a severe disease in patients with COPD and COVID-19. Zhao29 studied 11 case-series with a total number of 2002 patients. The pooled OR of COPD and the development of a severe form of disease were 4.38. Current smoker had an OR of 1.98. Alqahtani14 included 2473 patients in their review (14 out of 15 studies were from China). COPD patients and smokers were at higher risk of more severe disease, but prevalence rates of COPD patients were lower than in our study (2% vs. 8.3%). Our findings show that a prior diagnosis of lung disease, compared with those without respiratory diseases, is a prognostic factor for survival, after adjusting for confounders.
Predictors of mortality in patients with lung diseases pointed out some clinically relevant conclusions. Older age, use of home oxygen therapy, acute respiratory failure upon admission and elevated D-dimer were associated with an increased mortality risk. Also they had more comorbidities as diabetes, hypertension, obesity, chronic heart failure and previous immunosuppressive treatments that may explain in part an increased risk of mortality. Moreover, the use of systemic steroids during hospitalization increased the risk of dying in these patients which may be a bias effect in a more severe form of disease as a preliminary report of a study30 has shown a protective role of dexamethasone. In a Cox regression analysis, the only protective effect was found with gender (female versus male) and the use of Q/HCQ, which almost halved the hazard of dying. This last finding is controversial because results from studies are contradictory as one31 has shown no effect of Q/HCQ about mortality while another32 has presented a reduction of viral load in patients treated with Q/HCQ.
This study has several strengths, including a large size, immediacy, high data completion with relatively few missing variables, and data collection from diverse centers and countries. However, a number of potential limitations can be discussed. First, it has a retrospective observational design from several centers in 6 countries. Any source of selection or information bias, or confounding in collecting data cannot be ruled out, but at the same time give us a real view of management and prognosis of COVID-19 patients in an international scenario. Second, data is only about hospitalized patients with COVID-19, so we may lose information regarding milder forms of the disease. Third, results are based in a post hoc analysis and information generated would not be as robust as a prospective cohort or a clinical trial, so we have to consider efficacy of administered treatments with caution. Fourth, some patients with lung diseases have other comorbidities that may bias the increased risk of mortality. Others include, data collection mostly by non-pneumologists therefore without any a priori interest on respiratory disease, the universal underdiagnosis of lung diseases, absence of differential diagnosis and overlap of individual respiratory conditions. Results are on short-term mortality, so no exploration was conducted beyond 30 days post admission. Our aim was to generate hypotheses and to compare ours with other cohorts, but new studies should be done trying to analyze survival patterns and complications in patients with different lung diseases and COVID-19.
ConclusionsIn this large, international registry, 22% of patients hospitalized for COVID-19 had a previous lung disease, being COPD the most prevalent diagnosis (8% of the registry). Having a lung disease is a risk factor for mortality in patients with infection for SARS-CoV-2 and a higher mortality is described in our cohort. Older age, male gender, home oxygen therapy, and respiratory failure on admission were associated with an increased mortality. Efforts must be done to identify these patients upon admission to set measures to improve their clinical outcomes.
Author's contributionsJS-C had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis including any adverse effects. JS-C and IJN-G were involved in the design of the study. JBS, JACA were involved in data analysis. All authors were involved in the interpretation of the data and in the writing and critical review of the manuscript.
Conflicts of interestAll authors declare no competing interests.
Cardiovascular Excellence SL, for their essential support in the database and HOPE webpage. To all HOPE COVID-19 investigators, scientific committee and collaborators (Supplement; e-Table 5–7).