Blood eosinophil counts (BEC) have been related to the severity of bronchiectasis and its response to inhaled corticosteroids. However, only the baseline BEC has been used to assess this relationship and it is known that BEC could change over time. The objective of this study is to analyse the association of persistent eosinophilia or eosinopenia with outcomes in bronchiectasis.
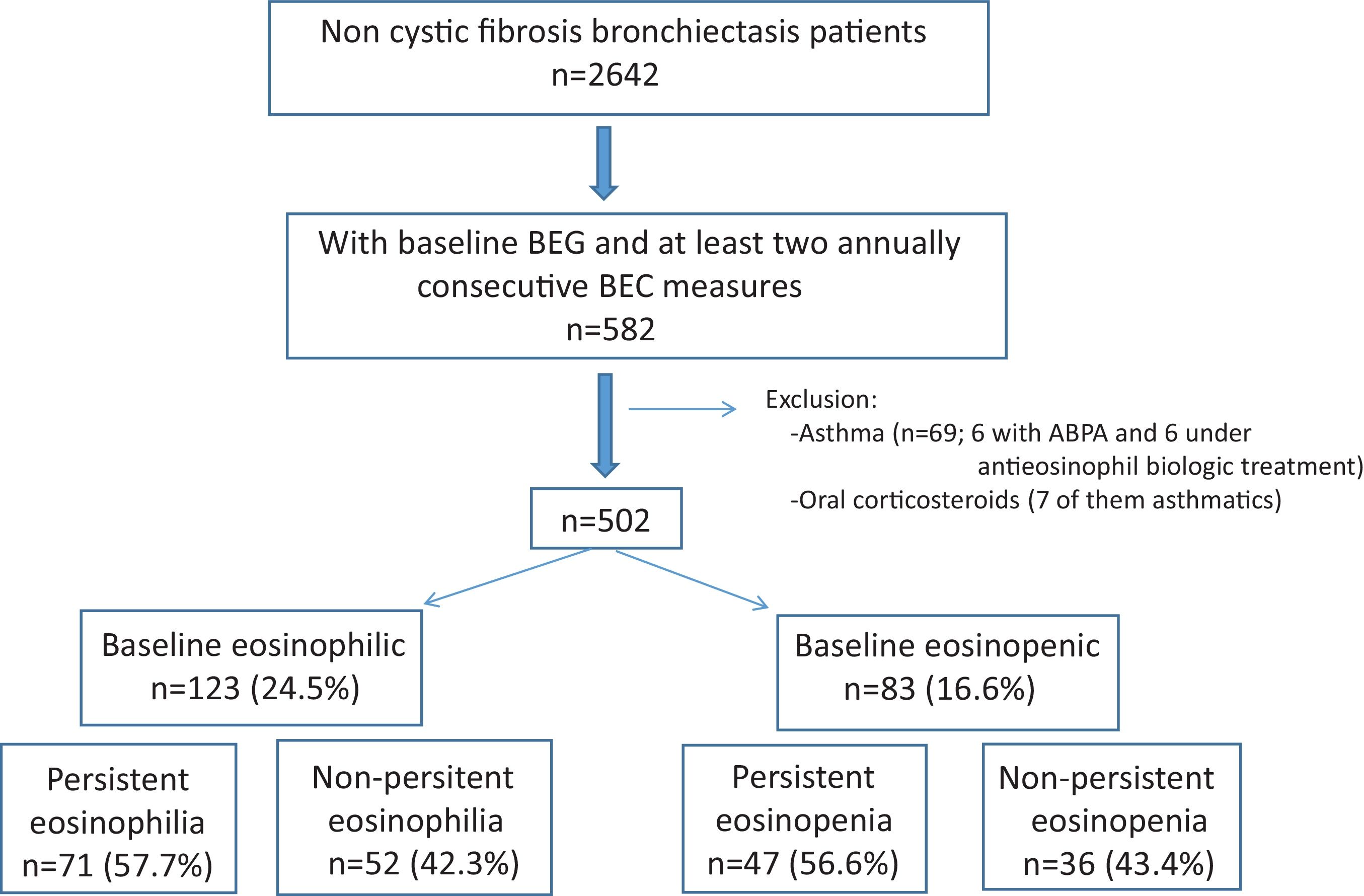
MethodsMulticentre, prospective and observational study from 43 centres in Spain derived from the Spanish Bronchiectasis Registry (RIBRON). Asthma and anti-eosinophil treatments were excluded. Patients with at least two yearly BEC measures (including the baseline measure) were included. Persistent eosinophilia (at least 300cells/μL) or persistent eosinopenia (less than 100cells/μL) were defined as the persistence in the same eosinophil group after three yearly measures (being the baseline the first measure).
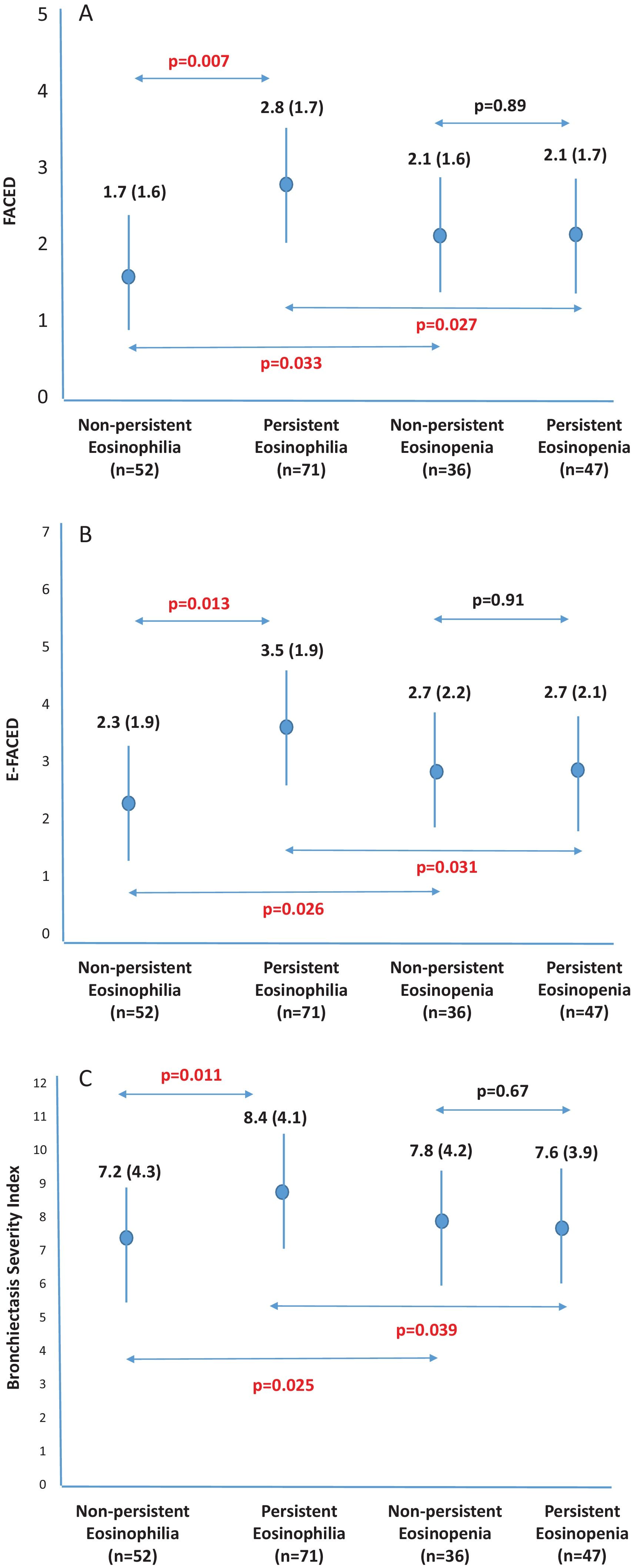
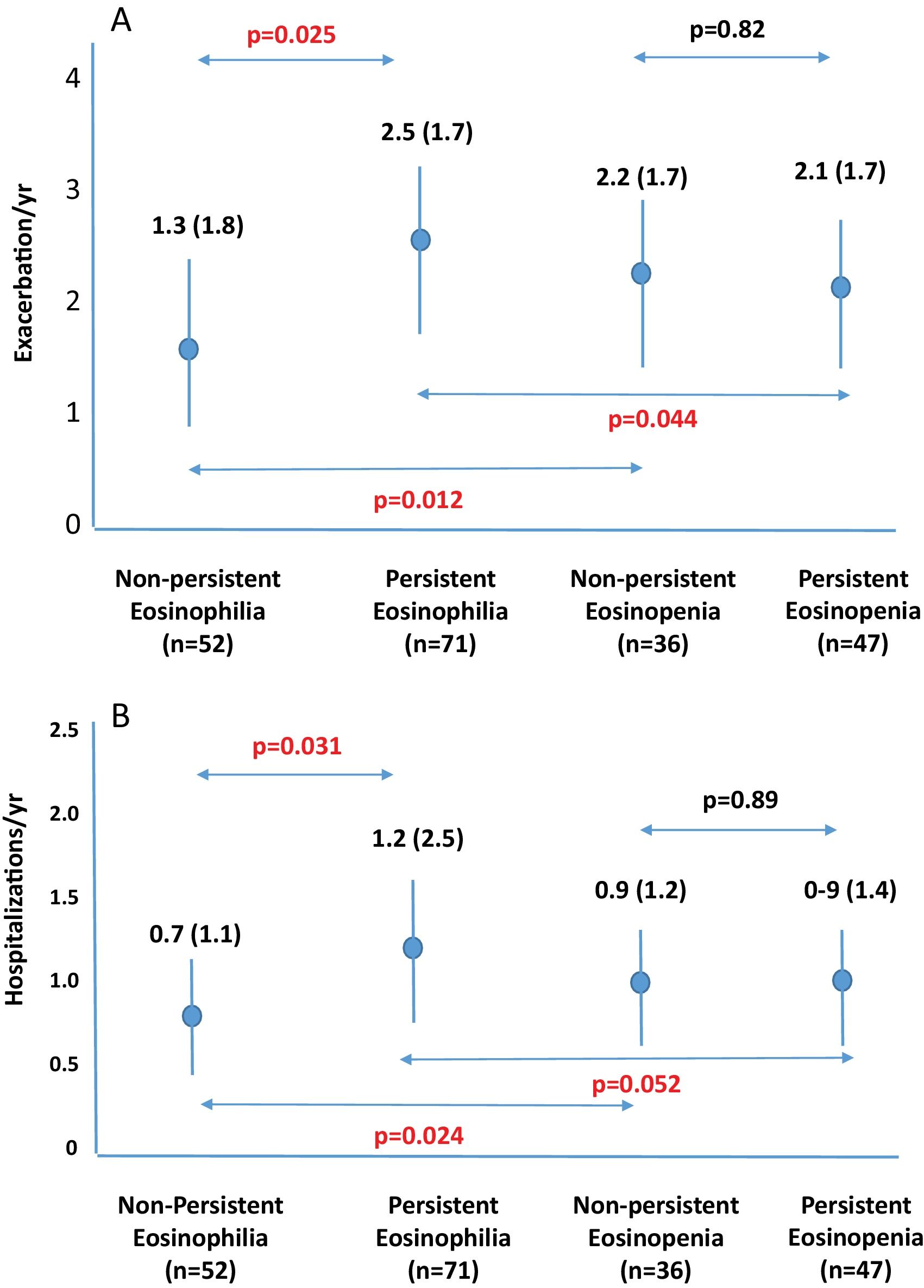
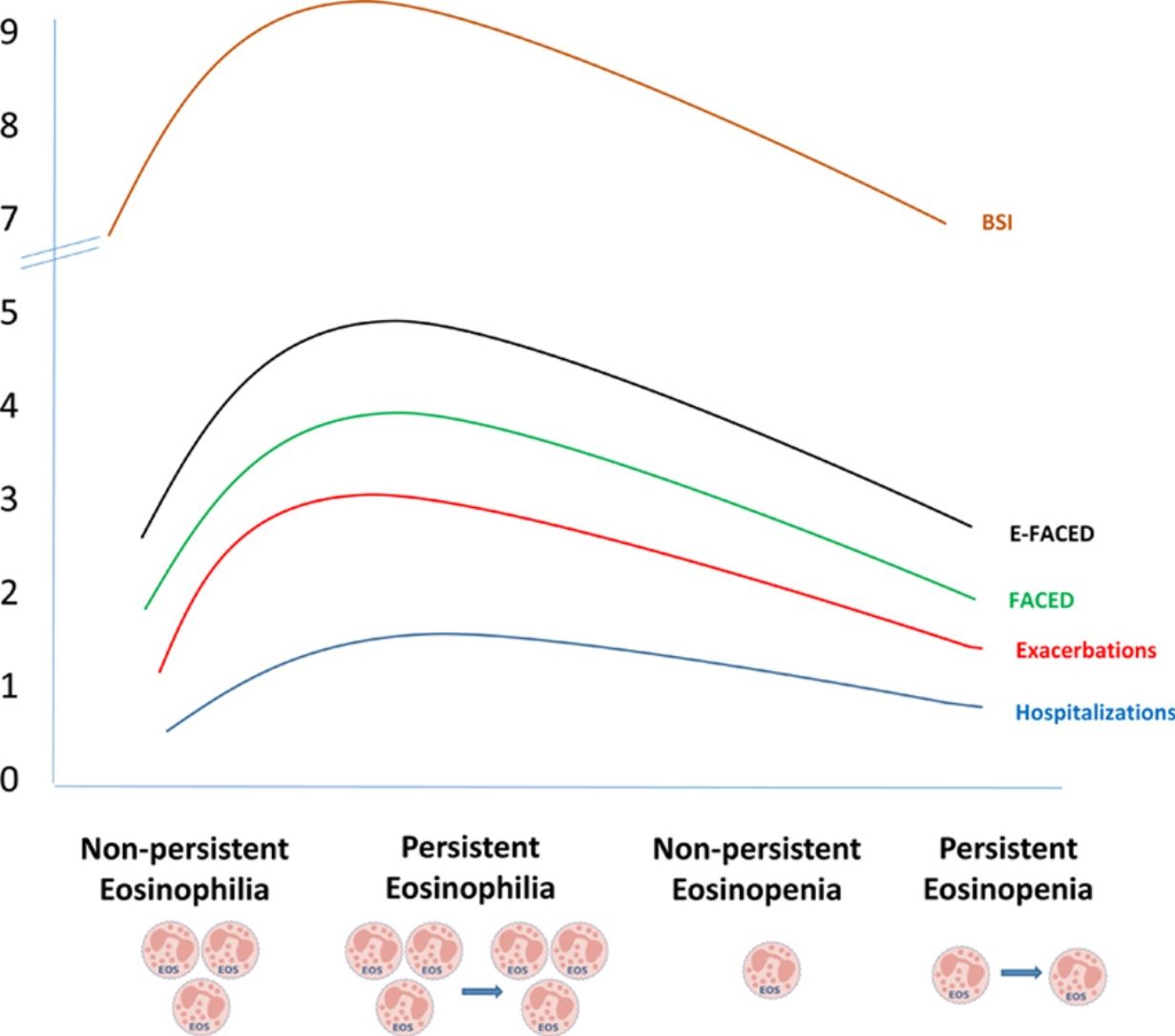
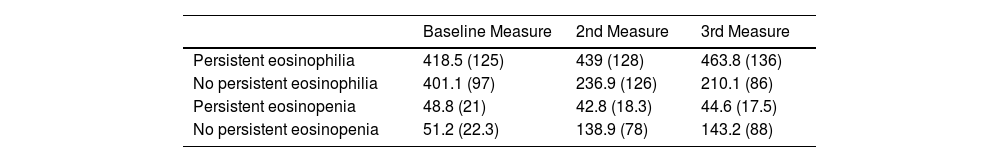
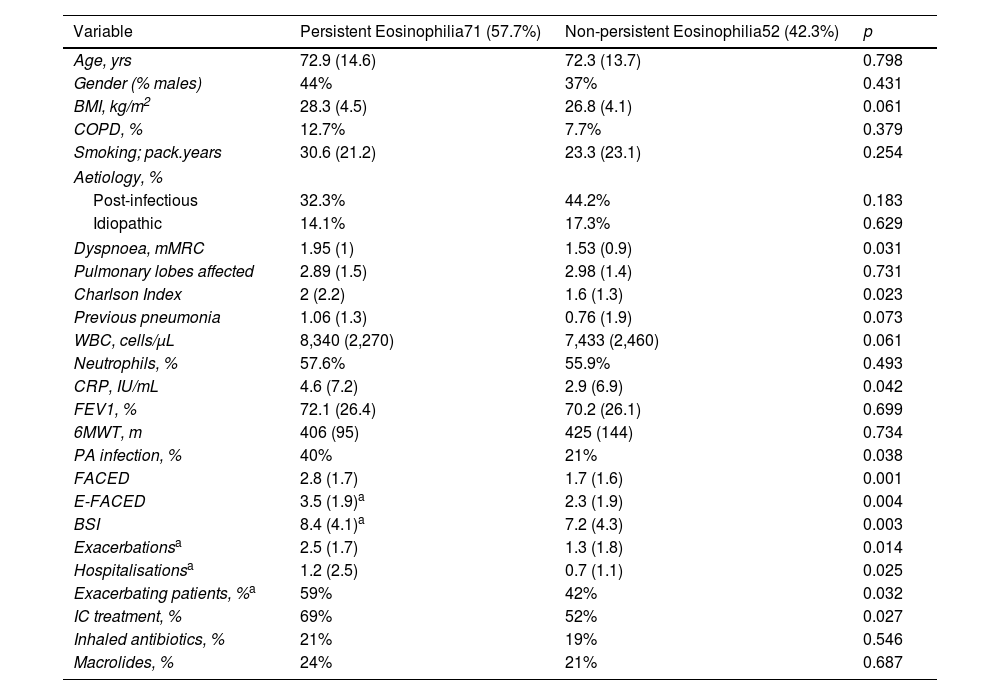
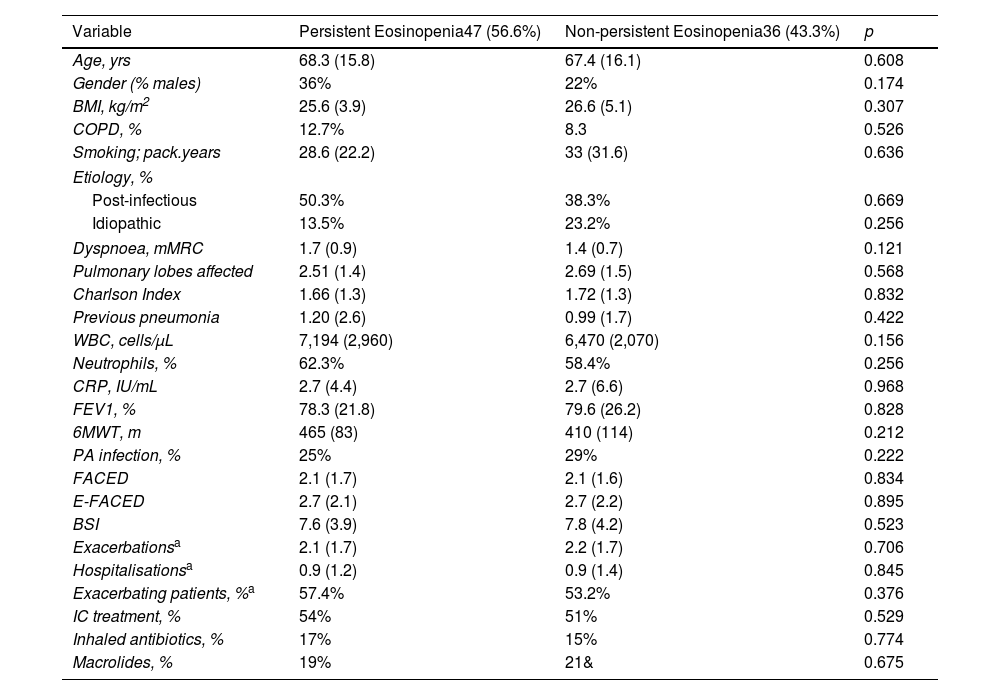
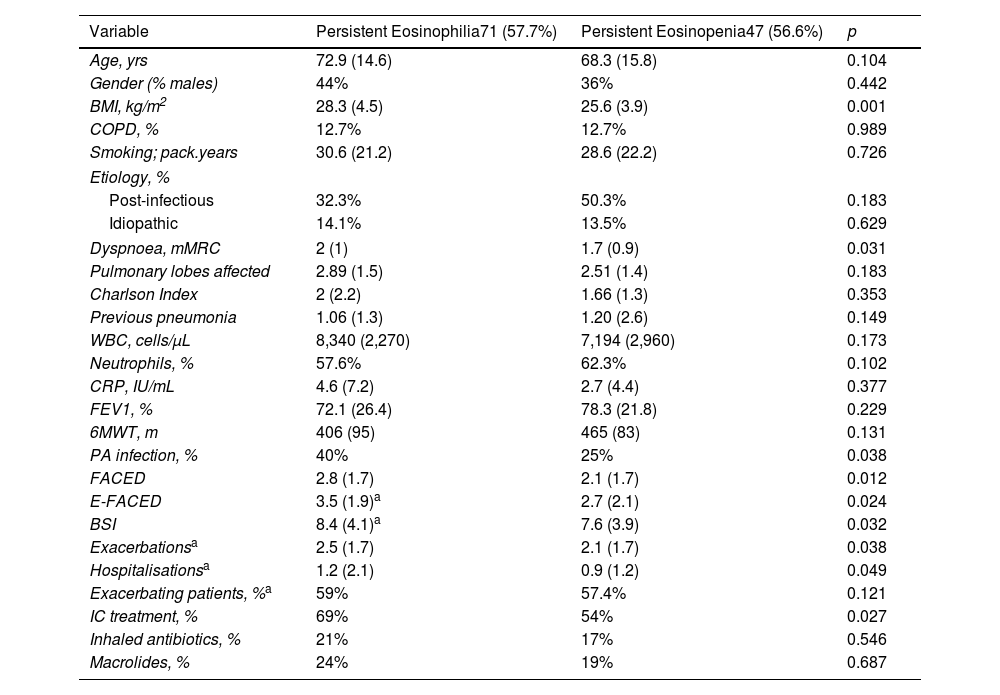
ResultsFive hundred two patients with at least three BEC measures were included; 24.5% and 16.6% presented baseline eosinophilia or eosinopenia, respectively. Of these, 57.7% and 56.6% presented persistent eosinophilia and eosinopenia, respectively. Patients with persistent eosinophilia presented greater severity and a higher number/greater severity of exacerbations than those with non-persistent eosinophilia and those with persistent or non-persistent eosinopenia. Finally, patients with non-persistent eosinopenia presented more severity and a higher number/greater severity of exacerbations than those with non-persistent eosinophilia.
ConclusionWhen only the baseline BEC was taken into account, patients with eosinopenia presented greater severity than those with eosinophilia. However, patients with persistent eosinophilia presented greater severity than those with persistent eosinopenia. Monitoring the BEC seems to be important in bronchiectasis.