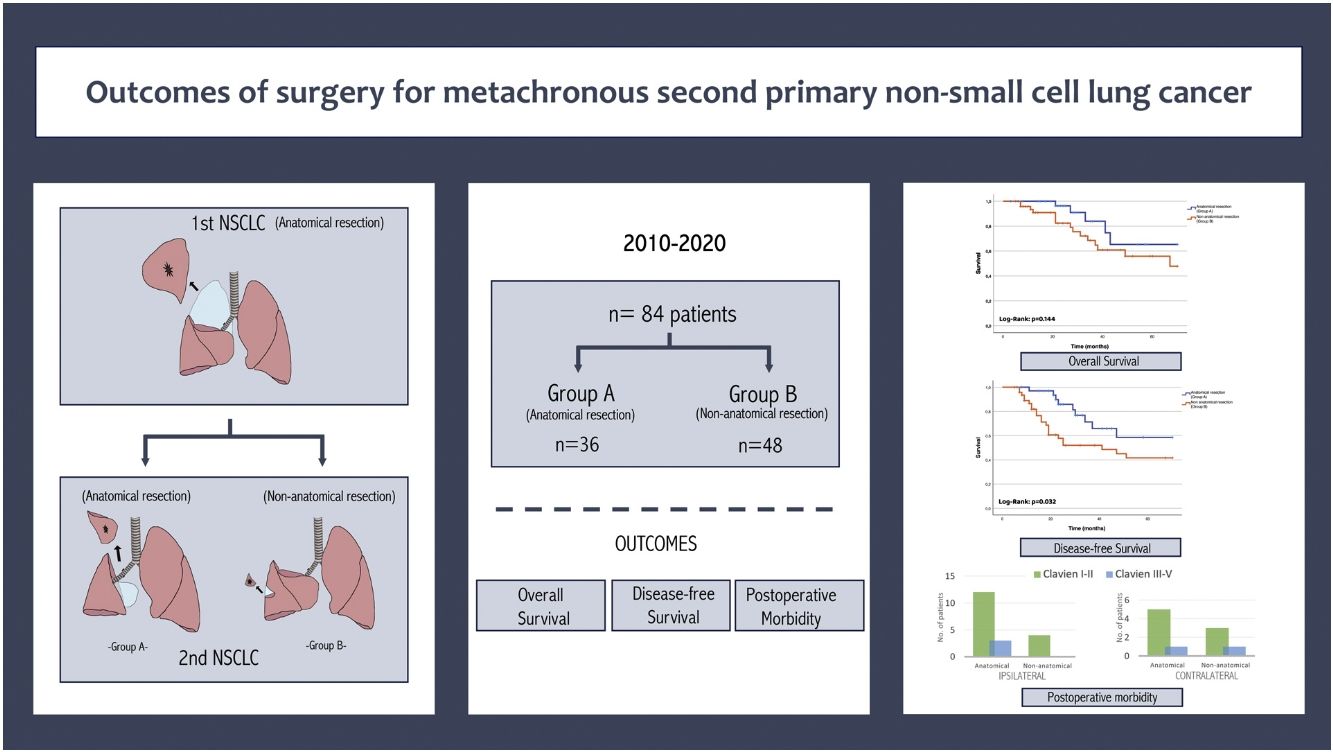
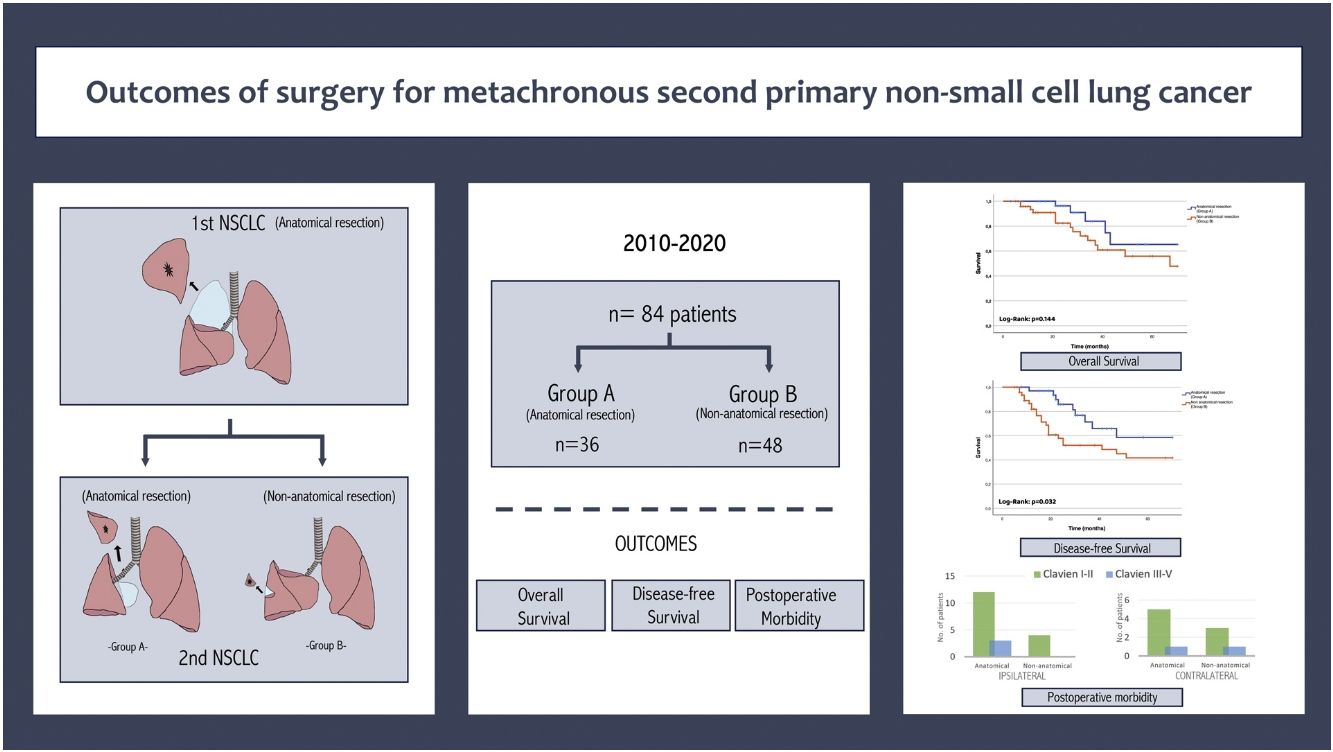
The optimal surgical approach for second primary metachronous lung cancer (MPLC) remains unclear. Our aim is to evaluate the morbidity and prognostic value based on the extent of surgical resection in MPLC.
MethodsRetrospective study of 84 patients with a history of anatomical resection for lung cancer and MPLC surgically treated between January 2010 and December 2020.
ResultsThe interval between the initial primary tumor and the second was 50.38±32.89 months. The second resection was contralateral in 43 patients (51.2%) and ipsilateral in 41 (48.8%). Thirty-six patients (42.9%) underwent a second anatomical resection, and in 48 patients (57.1%), it was non-anatomical. Postoperative complications were observed in 29 patients (34.5%) after the second lung resection. According to the Clavien-Dindo classification, 95.2% were mild (Clavien-Dindo I–II), and a single patient died (1.2%) in the postoperative period (Grade V). Prolonged air leak (p=0.037), postoperative arrhythmias (p=0.019) and hospital stay showed significant differences depending on the extent of surgery in ipsilateral resections. The main histological type was adenocarcinoma (47.6%) and the median tumor size was 17.74±11.74mm. The overall survival was 58.07 months (95% CI 49.29–66.85) for patients undergoing anatomical resection and 50.97 months (95% CI 43.31–58.63) for non-anatomical without significant differences (p=0.144). The disease-free survival after the second surgery was 53.75 months (95% CI 45.28–62.23) for anatomical resection and 41.34 months (95% CI 33.04–49.65) for non-anatomical group.
ConclusionSecond anatomical resections provide good long-term outcomes and have been shown to provide better disease-free survival compared to non-anatomical resections in properly selected patients.
After the improvements in prognosis that have occurred in recent decades in surgically treated non-small cell lung cancer (NSCLC) patients, the risk of developing a second metachronous primary lung cancer (MPLC) after complete resection of the initial tumor increases over time, estimated at approximately 1–2% per patient/year.1,2 However, due to the improvement of current treatments and follow-up protocols, an increase in the survival of NSCLC patients is observed,3 so, these new primary tumors often present as early-stage disease, which increases the likelihood of a second complete resection.4
In 1998 Johnson et al.,1 reviewed the outcomes of patients with MPLC, describing a 5-year overall survival of only 20%. As a result, second resections were limited for years with the pretext that they may not be as favorable as the initial resection. It was not until 2003 when it was suggested that surgery was entirely feasible in up to two-thirds of patients with MPLC.5 Furthermore, on occasions, the difficulty in distinguishing between a second metachronous lung cancer and a recurrence of the previous tumor further hinders these patients’ access to optimal treatment, which could be with curative intent.
Despite surgery is considered the gold standard for the treatment of early-stage NSCLC,6 given the scarcity of scientific evidence on the management of MPLC, a standardized treatment for these second tumors has not yet been established, leading to the existence of different therapeutic options available.
Although some studies indicate that surgery is the first-line treatment modality for patients with MPLC,7 there is no consensus on whether anatomic resection is better for these patients, despite the frequent presence of dense adhesions and hilar fibrosis, which require challenging surgical procedures and increase postoperative morbidity. Alternatively, a non-anatomic resection that allows for greater preservation of lung parenchyma may be preferable in patients with typically advanced age, frequent comorbidity, and limited cardiopulmonary functional reserve. This hypothesis is supported by recent studies that suggest that sublobar resections are an adequate treatment for early-stage NSCLC.8,9
The objective of the present study is to review the outcomes and prognostic value related to surgical resection in MPLC in relation to the extent of the performed resection (anatomical or non-anatomical resection). Overall survival and disease-free survival will be analyzed and compared as primary outcomes for each type of resection, aiming to identify significant differences in terms of efficacy and prognosis, as well as to analyze the morbidity and mortality associated with these second surgeries.
MethodsStudy PopulationRetrospective cohort study of patients who underwent a second lung resection for NSCLC between January 2010 and December 2020. The study included patients who had previously undergone an anatomical resection with lymphadenectomy and met the Martini–Melamed criteria10 for a second primary tumor: a different histology from the first neoplasm, or the same histology if there was a disease-free interval of at least 2 years between the two tumors; development of a new tumor from a carcinoma in situ or appearance of the second tumor in a different lobe or lung without evidence of common positive lymph nodes or extrapulmonary metastases.
Patients whose histology was inconclusive between primary tumor or extrathoracic metastasis, those with lymph node involvement or without lymphadenectomy, and patients lost during follow-up were excluded. Demographic variables, preoperative comorbidity, surgical procedure performed, postoperative evolution, histology, and survival were collected. The tumor stage was unified according to the 8th edition of the TNM classification for lung cancer. As part of the preoperative study, all patients underwent thoracic and abdominal computed tomography, positron emission tomography, and a complete respiratory functional study. Mediastinal staging was performed according to the criteria established by the European Society of Thoracic Surgery (ESTS).11
All patients had undergone a complete anatomical lung resection for CPCNP according to the IASLC criteria12 in the first surgery. For the second surgery, patients were classified into two groups based on the type of resection performed: Group A: anatomical resection (lobectomy, bilobectomy, or anatomical segmentectomy) and Group B: non-anatomical resection (wedge resection). Any adverse event occurring during the postoperative hospital stay or within the 90 days following the surgical intervention was considered a postoperative complication. Postoperative complications were defined according to the consensus document of the North American (STS) and European (ESTS) Thoracic Surgeons Societies13 and classified according to the Clavien-Dindo classification14 as either mild (Grade I and II) or severe (Grade IIIa to V).
For the survival study, the interval between the first and second lung resections was defined as disease-free survival 1 (DFS-1), and in those patients who developed a new tumor or disease recurrence after the second surgical intervention, this period was referred to as disease-free survival 2 (DFS-2).
The study was conducted following the STROBE guidelines for observational studies (Strengthening the Reporting of Observational studies in Epidemiology). The study protocol (identification number: PI 20-1947) received full approval from both the local institutional research review committee and the clinical research ethics committee. The patients’ data were collected in an anonymized and encrypted database in accordance with the recommendations of the Personal Data Protection and Patient Autonomy Law.
Statistical AnalysisThe descriptive analysis was conducted using absolute frequencies and percentages for categorical variables or mean and standard deviation for numeric variables. Univariate analysis was performed using the Pearson's χ2 test or Fisher's exact test when the conditions for application were not met for categorical variables, and the Student's T test for continuous variables. Variables with p<0.05 in the univariate Cox regression model were entered into the stepwise Cox analysis to evaluate independent factors. The survival rates were calculated using the Kaplan–Meier method and expressed with mean and standard deviation too. Differences in survival were compared using the Log-Rank and Cox tests. A p-value <0.05 was considered significant. Statistical analysis was conducted using the SPSS V.27 software package (IBM Corp, Chicago, IL, USA).
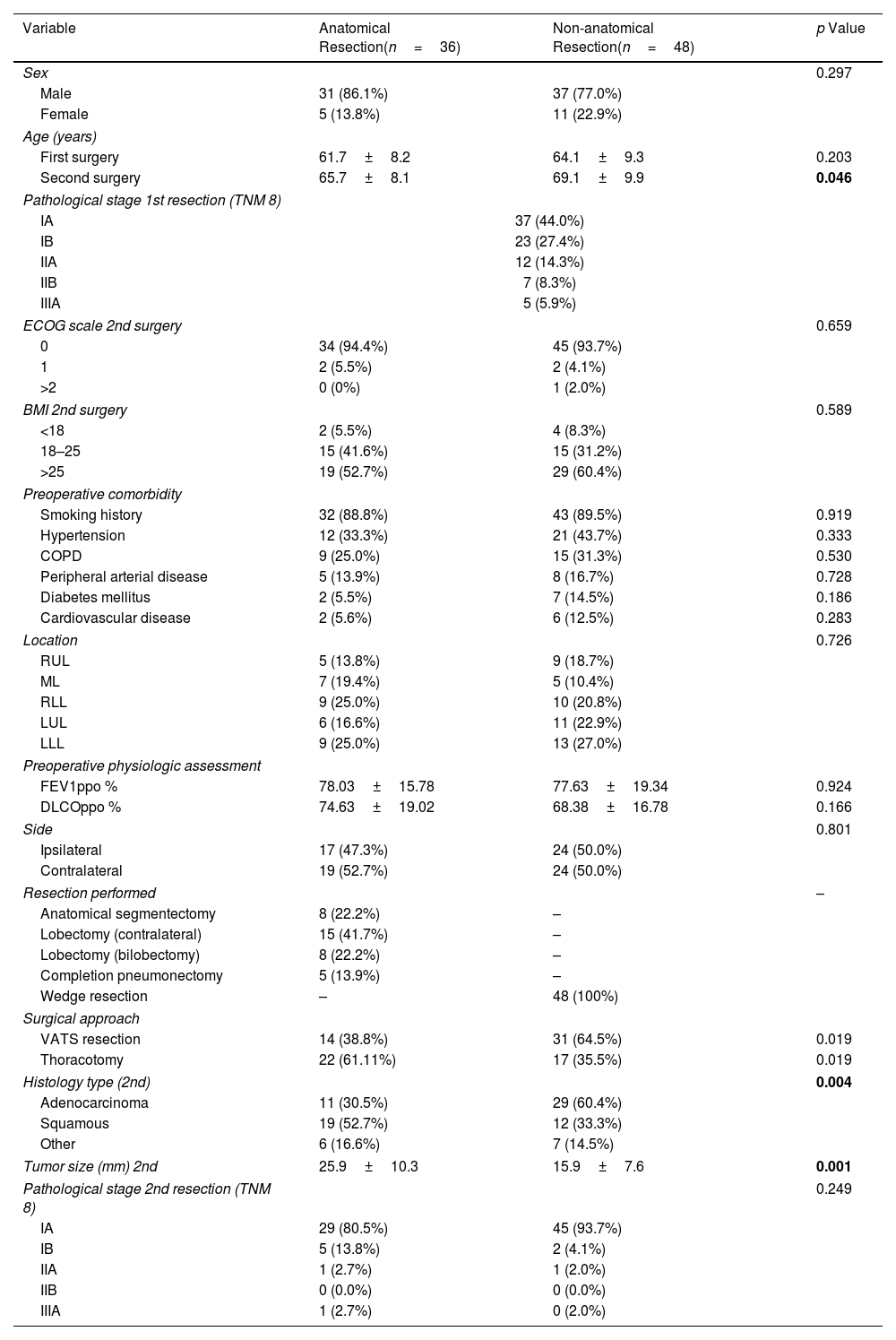
ResultsA total of 84 patients with a history of anatomical lung resection for NSCLC and who underwent a second primary lung tumor resection according to the Martini–Melamed criteria were analyzed. The study group consisted of 68 males (81%) and 16 females, with a mean age at the time of the first intervention of 62.7±8.7 years and at the second intervention of 67.1±9.0 years. The main perioperative clinical characteristics of the series are summarized in Table 1.
Demographics and Characteristics of All MPLC Patients (2nd Resection) According to the Extension of the Resection Performed.
| Variable | Anatomical Resection(n=36) | Non-anatomical Resection(n=48) | p Value |
|---|---|---|---|
| Sex | 0.297 | ||
| Male | 31 (86.1%) | 37 (77.0%) | |
| Female | 5 (13.8%) | 11 (22.9%) | |
| Age (years) | |||
| First surgery | 61.7±8.2 | 64.1±9.3 | 0.203 |
| Second surgery | 65.7±8.1 | 69.1±9.9 | 0.046 |
| Pathological stage 1st resection (TNM 8) | |||
| IA | 37 (44.0%) | ||
| IB | 23 (27.4%) | ||
| IIA | 12 (14.3%) | ||
| IIB | 7 (8.3%) | ||
| IIIA | 5 (5.9%) | ||
| ECOG scale 2nd surgery | 0.659 | ||
| 0 | 34 (94.4%) | 45 (93.7%) | |
| 1 | 2 (5.5%) | 2 (4.1%) | |
| >2 | 0 (0%) | 1 (2.0%) | |
| BMI 2nd surgery | 0.589 | ||
| <18 | 2 (5.5%) | 4 (8.3%) | |
| 18–25 | 15 (41.6%) | 15 (31.2%) | |
| >25 | 19 (52.7%) | 29 (60.4%) | |
| Preoperative comorbidity | |||
| Smoking history | 32 (88.8%) | 43 (89.5%) | 0.919 |
| Hypertension | 12 (33.3%) | 21 (43.7%) | 0.333 |
| COPD | 9 (25.0%) | 15 (31.3%) | 0.530 |
| Peripheral arterial disease | 5 (13.9%) | 8 (16.7%) | 0.728 |
| Diabetes mellitus | 2 (5.5%) | 7 (14.5%) | 0.186 |
| Cardiovascular disease | 2 (5.6%) | 6 (12.5%) | 0.283 |
| Location | 0.726 | ||
| RUL | 5 (13.8%) | 9 (18.7%) | |
| ML | 7 (19.4%) | 5 (10.4%) | |
| RLL | 9 (25.0%) | 10 (20.8%) | |
| LUL | 6 (16.6%) | 11 (22.9%) | |
| LLL | 9 (25.0%) | 13 (27.0%) | |
| Preoperative physiologic assessment | |||
| FEV1ppo % | 78.03±15.78 | 77.63±19.34 | 0.924 |
| DLCOppo % | 74.63±19.02 | 68.38±16.78 | 0.166 |
| Side | 0.801 | ||
| Ipsilateral | 17 (47.3%) | 24 (50.0%) | |
| Contralateral | 19 (52.7%) | 24 (50.0%) | |
| Resection performed | – | ||
| Anatomical segmentectomy | 8 (22.2%) | – | |
| Lobectomy (contralateral) | 15 (41.7%) | – | |
| Lobectomy (bilobectomy) | 8 (22.2%) | – | |
| Completion pneumonectomy | 5 (13.9%) | – | |
| Wedge resection | – | 48 (100%) | |
| Surgical approach | |||
| VATS resection | 14 (38.8%) | 31 (64.5%) | 0.019 |
| Thoracotomy | 22 (61.11%) | 17 (35.5%) | 0.019 |
| Histology type (2nd) | 0.004 | ||
| Adenocarcinoma | 11 (30.5%) | 29 (60.4%) | |
| Squamous | 19 (52.7%) | 12 (33.3%) | |
| Other | 6 (16.6%) | 7 (14.5%) | |
| Tumor size (mm) 2nd | 25.9±10.3 | 15.9±7.6 | 0.001 |
| Pathological stage 2nd resection (TNM 8) | 0.249 | ||
| IA | 29 (80.5%) | 45 (93.7%) | |
| IB | 5 (13.8%) | 2 (4.1%) | |
| IIA | 1 (2.7%) | 1 (2.0%) | |
| IIB | 0 (0.0%) | 0 (0.0%) | |
| IIIA | 1 (2.7%) | 0 (2.0%) | |
BMI: body mass index; COPD: chronic obstructive pulmonary disease; RUL: right upper lobe, ML: middle lobe; RLL: right lower lobe; LUL: left upper lobe; LLL: left lower lobe; FEV1ppo: predicted postoperative forced expiratory volume at one second; DLCOppo: predicted postoperative diffusing capacity for carbon monoxide.
Bold values are the statistically significant results.
The DFS-1 between the initial primary tumor and the second primary tumor was 50.38±32.89 months (range 6–136 months). Preoperative respiratory function study showed mean values of FEV1ppo 97.83±20.96 and DLCOppo 86.89±23.57 for the first lung resection and 77.81±17.73 and 71.93±18.22 respectively on reevaluation prior to the second intervention (p=0.001). In 43 patients (51.2%), the second resection was contralateral and in 41 (48.8%) it was ipsilateral. Regarding the extent of lung resection, 36 patients (42.9%) underwent a second anatomical resection (ipsilateral: lobectomy 47.0%, segmentectomy 23.5% and completion pneumonectomy in 29.41%; contralateral: lobectomy 65.2% and segmentectomy 17.4%) and in 48 patients (57.1%) the resection performed was non-anatomical (wedge).
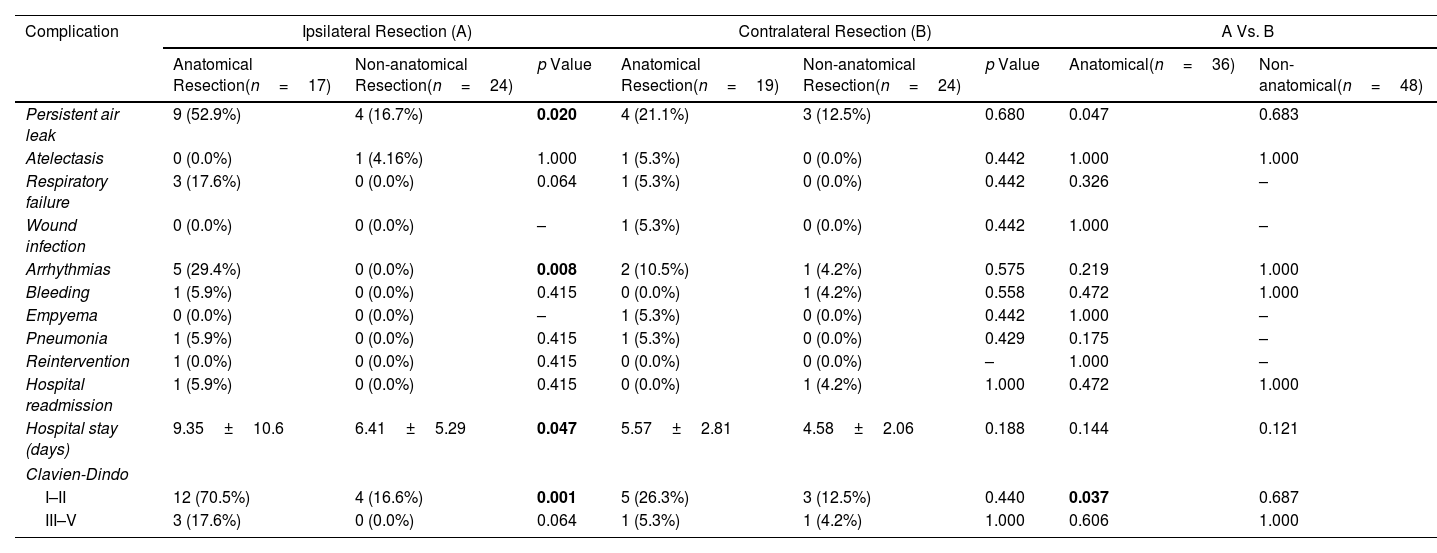
Postoperative complications after the second lung resection were seen in 29 patients of the series (34.5%). Among those recorded, the most frequent cause of major morbidity was prolonged air leak (defined as persistence beyond the 5th postoperative day), observed in 20 patients (23.8%). The complete list of postoperative complications is shown in Table 2. The relationship between the observed complications and the extension and laterality of the performed resection was analyzed. Only prolonged air leak (p=0.020), appearance of postoperative arrhythmias (p=0.019) and hospital stay (0.047) showed significant differences between anatomical resections vs. non-anatomical resections performed ipsilaterally. Conversely, in contralateral resections, no differences were observed. Similarly, only persistent air leak showed significant differences (p=0.047) among different anatomical resections regardless of laterality.
Main Morbidity Causes Observed After the Second Intervention According to the Extension and Laterality of the Resection Performed.
| Complication | Ipsilateral Resection (A) | Contralateral Resection (B) | A Vs. B | |||||
|---|---|---|---|---|---|---|---|---|
| Anatomical Resection(n=17) | Non-anatomical Resection(n=24) | p Value | Anatomical Resection(n=19) | Non-anatomical Resection(n=24) | p Value | Anatomical(n=36) | Non-anatomical(n=48) | |
| Persistent air leak | 9 (52.9%) | 4 (16.7%) | 0.020 | 4 (21.1%) | 3 (12.5%) | 0.680 | 0.047 | 0.683 |
| Atelectasis | 0 (0.0%) | 1 (4.16%) | 1.000 | 1 (5.3%) | 0 (0.0%) | 0.442 | 1.000 | 1.000 |
| Respiratory failure | 3 (17.6%) | 0 (0.0%) | 0.064 | 1 (5.3%) | 0 (0.0%) | 0.442 | 0.326 | – |
| Wound infection | 0 (0.0%) | 0 (0.0%) | – | 1 (5.3%) | 0 (0.0%) | 0.442 | 1.000 | – |
| Arrhythmias | 5 (29.4%) | 0 (0.0%) | 0.008 | 2 (10.5%) | 1 (4.2%) | 0.575 | 0.219 | 1.000 |
| Bleeding | 1 (5.9%) | 0 (0.0%) | 0.415 | 0 (0.0%) | 1 (4.2%) | 0.558 | 0.472 | 1.000 |
| Empyema | 0 (0.0%) | 0 (0.0%) | – | 1 (5.3%) | 0 (0.0%) | 0.442 | 1.000 | – |
| Pneumonia | 1 (5.9%) | 0 (0.0%) | 0.415 | 1 (5.3%) | 0 (0.0%) | 0.429 | 0.175 | – |
| Reintervention | 1 (0.0%) | 0 (0.0%) | 0.415 | 0 (0.0%) | 0 (0.0%) | – | 1.000 | – |
| Hospital readmission | 1 (5.9%) | 0 (0.0%) | 0.415 | 0 (0.0%) | 1 (4.2%) | 1.000 | 0.472 | 1.000 |
| Hospital stay (days) | 9.35±10.6 | 6.41±5.29 | 0.047 | 5.57±2.81 | 4.58±2.06 | 0.188 | 0.144 | 0.121 |
| Clavien-Dindo | ||||||||
| I–II | 12 (70.5%) | 4 (16.6%) | 0.001 | 5 (26.3%) | 3 (12.5%) | 0.440 | 0.037 | 0.687 |
| III–V | 3 (17.6%) | 0 (0.0%) | 0.064 | 1 (5.3%) | 1 (4.2%) | 1.000 | 0.606 | 1.000 |
Bold values are the statistically significant results.
Complications were classified according to the Clavien-Dindo severity classification and 95.2% of these complications were classified as mild (Clavien-Dindo I–II), with only a single patient died (1.2%) in the early postoperative period due to post-surgical complications (Grade V). A higher number of “mild” complications were observed among ipsilateral procedures compared to contralateral resections, especially in anatomical surgeries (p=0.037), and in relation to non-anatomical ipsilateral resections (p=0.001). However, no differences were found regarding the laterality or extent of the performed surgery concerning “severe” complications (Clavien-Dindo >3).
The main histological type was adenocarcinoma (40 patients, 47.6%). Regarding the pathological stage in the second surgery, 74 cases (88.1%) were in stage I, 9 in stage II (10.7%), and 1 in stage III (1.2%). The median tumor size was 17.74±11.74mm.
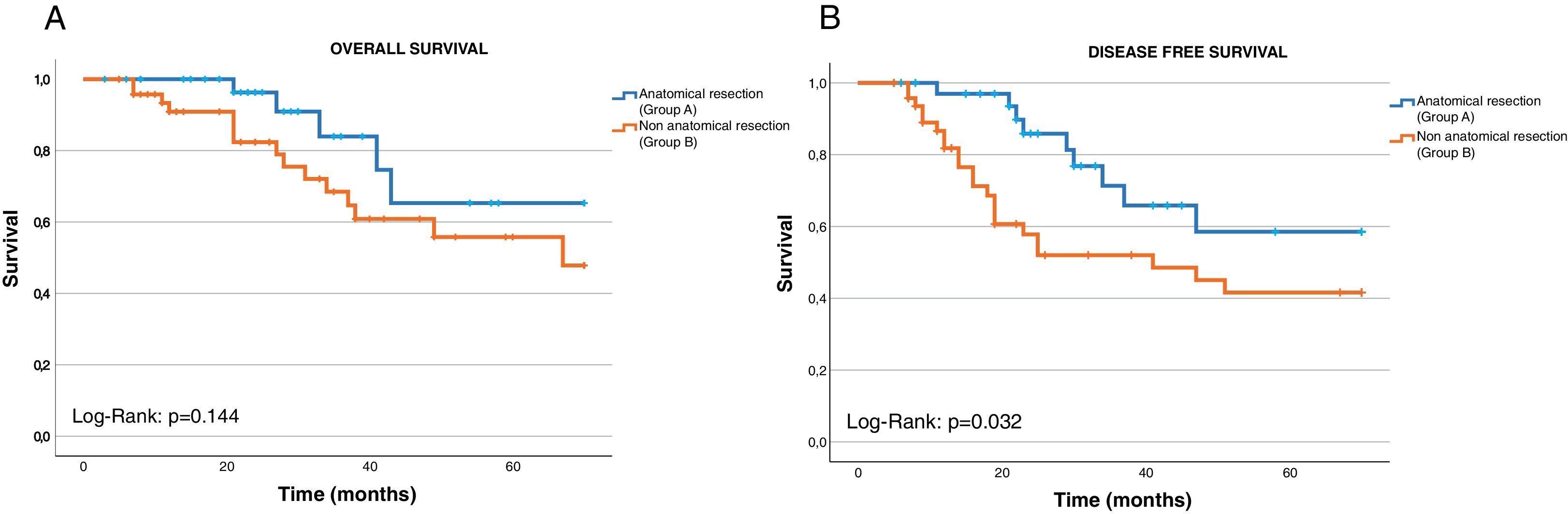
The median follow-up from the second surgery was 61 months (range 6–122 months). The 5-year overall survival rate (OS) was 63.09% and the DFI-2 was 64.28%. Survival was independently analyzed according to the type of resection performed in the second intervention. We observed that the OS was 58.07±4.48 months (95% CI 49.29–66.85) for patients undergoing anatomical resection (Group A) and 50.97±43.90 months (95% CI 43.31–58.63) for those undergoing non-anatomical resection (Group B). No statistically significant differences were observed in the Log-Rank test (p=0.144; Fig. 1A).
The DFS-2 was also analyzed, being 53.75±4.32 months (95% CI 45.28–62.23) in patients belonging to Group A and 41.34±4.23 months (95% CI 33.04–49.65) in those belonging to Group B. The Log-Rank test showed statistical association with this variable (p=0.032; Fig. 1B).
Based on univariate analysis, several factors were identified to have a negative impact on survival, such as tumor size >3cm (p=0.001), ECOG scale >2 (p=0.016), complications ≥Grade III (p=0.044), and squamous cell histology (p=0.022). On the other hand, factors recognized as negative for DFS-2 in the univariate study were the presence of preoperative comorbidity (p=0.022), non-anatomic resection (p=0.039), postoperative complications (p=0.031), squamous cell histology (p=0.045), and tumor size >3cm (p=0.015).
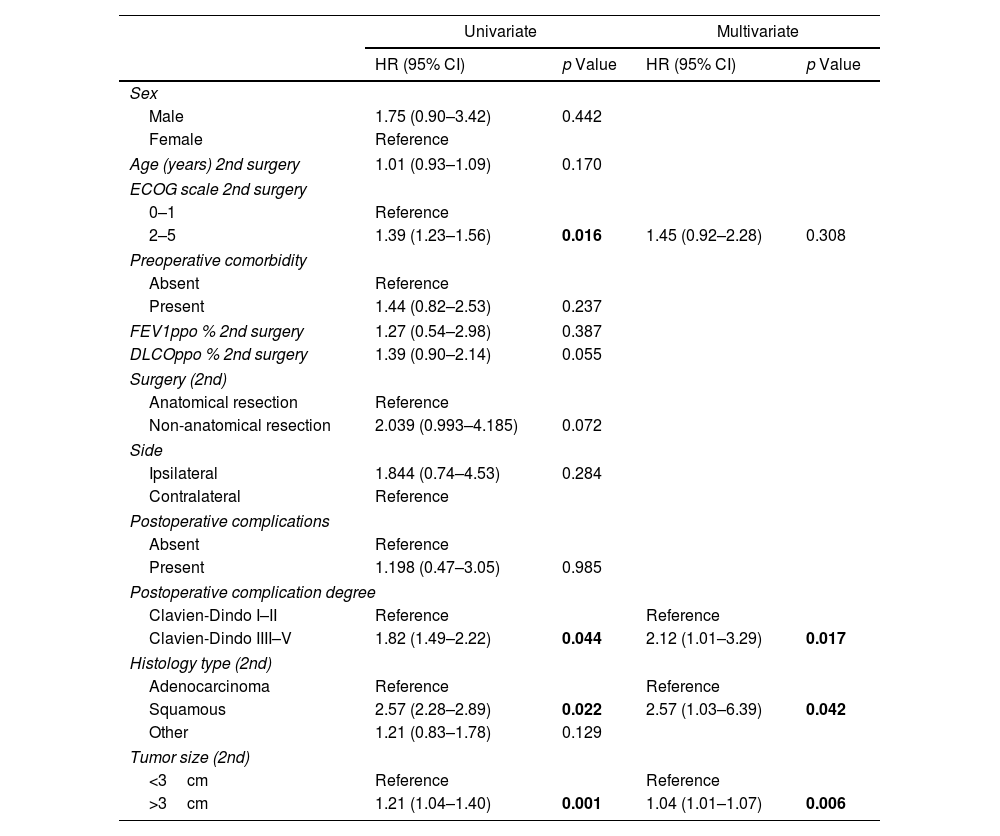
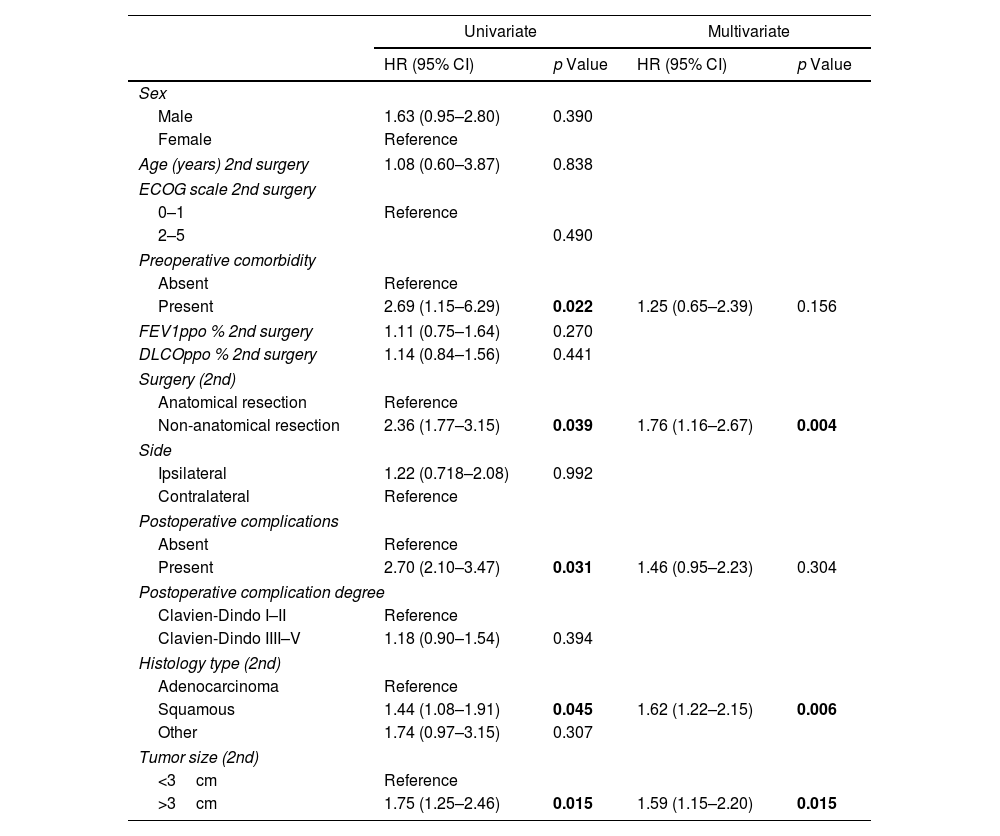
Stepwise Cox regression analysis was used, demonstrating that tumor size >3cm (HR 1.04; 95% CI 1.01–1.07; p=0.006), squamous histology (HR 2.57; 95% CI 1.03–6.39; p=0.042) and complications ≥Grade III (HR 2.12; 95% CI 1.01–3.29; p=0.017) were independent predictors of survival in multivariate analysis, while non-anatomical resection (HR 1.76; 95% CI 1.16–2.67; p=0.004), squamous histology (HR 1.62; 95% CI 1.22–2.15; p=0.006) and tumor size >3cm (HR 1.59; 95% CI 1.15–2.20; p=0.015) were shown to negatively influence DFS-2. The rest of the analysis can be observed in Tables 3 and 4.
Uni and Multivariate Analyses of Risk Factors for Overall Survival.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Sex | ||||
| Male | 1.75 (0.90–3.42) | 0.442 | ||
| Female | Reference | |||
| Age (years) 2nd surgery | 1.01 (0.93–1.09) | 0.170 | ||
| ECOG scale 2nd surgery | ||||
| 0–1 | Reference | |||
| 2–5 | 1.39 (1.23–1.56) | 0.016 | 1.45 (0.92–2.28) | 0.308 |
| Preoperative comorbidity | ||||
| Absent | Reference | |||
| Present | 1.44 (0.82–2.53) | 0.237 | ||
| FEV1ppo % 2nd surgery | 1.27 (0.54–2.98) | 0.387 | ||
| DLCOppo % 2nd surgery | 1.39 (0.90–2.14) | 0.055 | ||
| Surgery (2nd) | ||||
| Anatomical resection | Reference | |||
| Non-anatomical resection | 2.039 (0.993–4.185) | 0.072 | ||
| Side | ||||
| Ipsilateral | 1.844 (0.74–4.53) | 0.284 | ||
| Contralateral | Reference | |||
| Postoperative complications | ||||
| Absent | Reference | |||
| Present | 1.198 (0.47–3.05) | 0.985 | ||
| Postoperative complication degree | ||||
| Clavien-Dindo I–II | Reference | Reference | ||
| Clavien-Dindo IIII–V | 1.82 (1.49–2.22) | 0.044 | 2.12 (1.01–3.29) | 0.017 |
| Histology type (2nd) | ||||
| Adenocarcinoma | Reference | Reference | ||
| Squamous | 2.57 (2.28–2.89) | 0.022 | 2.57 (1.03–6.39) | 0.042 |
| Other | 1.21 (0.83–1.78) | 0.129 | ||
| Tumor size (2nd) | ||||
| <3cm | Reference | Reference | ||
| >3cm | 1.21 (1.04–1.40) | 0.001 | 1.04 (1.01–1.07) | 0.006 |
FEV1ppo: predicted postoperative forced expiratory volume at one second; DLCOppo: predicted postoperative diffusing capacity for carbon monoxide.
Bold values are the statistically significant results.
Uni and Multivariate Analyses of Risk Factors for Disease-free Survival.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Sex | ||||
| Male | 1.63 (0.95–2.80) | 0.390 | ||
| Female | Reference | |||
| Age (years) 2nd surgery | 1.08 (0.60–3.87) | 0.838 | ||
| ECOG scale 2nd surgery | ||||
| 0–1 | Reference | |||
| 2–5 | 0.490 | |||
| Preoperative comorbidity | ||||
| Absent | Reference | |||
| Present | 2.69 (1.15–6.29) | 0.022 | 1.25 (0.65–2.39) | 0.156 |
| FEV1ppo % 2nd surgery | 1.11 (0.75–1.64) | 0.270 | ||
| DLCOppo % 2nd surgery | 1.14 (0.84–1.56) | 0.441 | ||
| Surgery (2nd) | ||||
| Anatomical resection | Reference | |||
| Non-anatomical resection | 2.36 (1.77–3.15) | 0.039 | 1.76 (1.16–2.67) | 0.004 |
| Side | ||||
| Ipsilateral | 1.22 (0.718–2.08) | 0.992 | ||
| Contralateral | Reference | |||
| Postoperative complications | ||||
| Absent | Reference | |||
| Present | 2.70 (2.10–3.47) | 0.031 | 1.46 (0.95–2.23) | 0.304 |
| Postoperative complication degree | ||||
| Clavien-Dindo I–II | Reference | |||
| Clavien-Dindo IIII–V | 1.18 (0.90–1.54) | 0.394 | ||
| Histology type (2nd) | ||||
| Adenocarcinoma | Reference | |||
| Squamous | 1.44 (1.08–1.91) | 0.045 | 1.62 (1.22–2.15) | 0.006 |
| Other | 1.74 (0.97–3.15) | 0.307 | ||
| Tumor size (2nd) | ||||
| <3cm | Reference | |||
| >3cm | 1.75 (1.25–2.46) | 0.015 | 1.59 (1.15–2.20) | 0.015 |
FEV1ppo: predicted postoperative forced expiratory volume at one second; DLCOppo: predicted postoperative diffusing capacity for carbon monoxide.
Bold values are the statistically significant results.
The optimal extent of surgical resection in patients with a second primary lung tumor is a challenge, especially for those with a history of previous anatomical resection, since in addition to preserving functional reserve, we must ensure the oncologic principles of lung cancer surgery.
In our study, we observed that although anatomical resections are associated with relatively greater overall survival than non-anatomical resections, these differences do not demonstrate statistically significant differences. These results are consistent with those published by other authors. Thus, Yang et al.,15 described lobectomy as a valid option for the treatment of MPLC, although non-anatomical resections could also be a useful alternative in tumors smaller than 2cm. Other authors have also concluded their work in favor of non-anatomical resection in MPLC,16,17 especially in tumors smaller than 15mm.18
Similarly, Lee et al.,19 in a study based on the Surveillance, Epidemiology and End Results (SEER) database, reported that lobectomy is comparable to sublobar resections for patients with early-stage MPLC. However, more recent studies20 have observed a higher cancer-specific mortality rate in non-anatomic resections compared to anatomical ones. On the other hand, Hattori et al.,21 have described that second anatomical resections are oncologically acceptable, although they are a clear significant predictor of postoperative morbidity, which requires exhaustive selection of these patients.
In our study, we detected an incidence of global complications in up to 34.5% of surgically treated patients, with the majority (95.2%) being mild in nature and only 4.8% major, with a single perioperative death (1.2%) recorded. We have observed that ipsilateral resections exhibit a higher proportion of postoperative complications, particularly among those undergoing second ipsilateral anatomical resections.
These data are similar to those reported in the literature. Shah et al.,22 for example, described a postoperative morbidity of 28%, with 6% of these being major, and 2% associated mortality. Abid et al.,17 in a study of morbidity in patients with MPLC, observed a complications rate similar to the first surgical intervention, with 36.5% of complications and no associated mortality. These rates of complications can escalate considerably in surgeries aimed at completing pneumonectomy, reaching up to 60% for major complications.23,24
Two recent studies stand out in this regard. The first study, conducted by Okazaki et al.,25 observed a high frequency of intra and postoperative complications, especially in the case of ipsilateral resections. However, they noted comparable survival between these patients and those who underwent anatomical resection without prior anatomical resection.
On the other hand, Zhao et al.,26 while describing similar prognostic characteristics between the primary tumor and the MLSC (multiple primary lung cancer), recommend avoiding completion pneumonectomy due to the high morbidity associated with this procedure, which results in a poorer prognosis in this subgroup of patients.
In our work, we have observed that although second resections, especially ipsilateral ones, are associated with a high morbidity rate, in general, these complications tend to be of mild nature, with less than 5% of complications categorized as severe. Therefore, based on our findings, we consider that an optimal pre-surgical patient selection is necessary for those who may benefit from a new surgical treatment with lower associated morbidity.
One of the main novelties that we bring in this study is the analysis of disease-free interval between the second surgically intervened neoplasm and the subsequent tumor recurrence. In this case, we have been able to demonstrate that patients treated with anatomical resections show significant differences compared to those treated with non-anatomical resections (53.75 months vs. 41.34 months; p=0.032). This aspect has been poorly analyzed in the medical literature and for this purpose, the research by Fourdrain et al.,27 stands out, who, although described a disease-free survival of 63.8% at 5 years, did not analyze possible differences based on surgical resection.
Similarly, Hattori et al.,21 described a 5-year disease-free survival (DFS) of 58.0%, and in the survival analysis, there was a higher DFS in favor of anatomical resections (77.9% vs. 67.5%), although they could not demonstrate that these differences were statistically significant. Sato et al.,16 on the other hand, analyzed the difference based on the type of resection performed without observing statistically significant differences, probably because most patients with non-anatomical resections had tumors smaller than 20mm and predominantly of lepidic growth pattern, a significantly higher number than those undergoing anatomical resection.
With the aim of identifying factors associated with better OS and DFS, univariate and multivariate analyses were performed. In the multivariate analysis, postoperative morbidity >Grade III and tumor size >3cm were associated with worse survival, while non-anatomical resection, as well as tumor size >3cm, were associated with worse DFS. Although gender and age have been previously identified as prognostic factors in previous studies,15,18 in our case, neither was associated with worse prognosis. Interestingly, in our series, we observed worse OS (HR 2.57, 95% CI 1.03–6.39) and DFS (HR 1.62, 95% CI 1.22–2.15) associated with squamous histology in the second resection, in line with other groups,15,28 so the presence of these factors may guide resection in certain selected patients.
Our study is limited by its retrospective and single-center nature, as well as the lack of randomization. Despite using the widely employed Martini–Melamed criteria to distinguish between a second lung cancer and metastasis, the absence of genetic and molecular characteristics to differentiate them more accurately may have unintentionally led to a selection bias of patients. Moreover, the limited sample size hinders the capacity to conduct further multivariate analyses, particularly in the context of ipsilateral resections.
We consider that in the future, the incorporation of alternative methodologies, such as advanced genetic and molecular profiling techniques, may aid in overcoming the limitations encountered in our investigation. Furthermore, given the significance of ipsilateral resections in postoperative morbidity, it could be interesting to focus future research specifically on these cases, even by focusing on the morbidity and mortality associated with different approaches.
To obtain a more comprehensive understanding of the effectiveness and validity of anatomical resection compared to non-anatomical resection in MPLC, future prospective and randomized studies are needed too to confirm the findings described in the present study, even in the context of multicenter studies that allow for a larger sample size, enabling comprehensive multivariate analyses, valuable information can be obtained regarding the outcomes and long-term effects of different resection techniques.
ConclusionSecond resections for MPLC are safe and provide good long-term survival. Performing an anatomical resection has been shown to provide better disease-free survival compared to a non-anatomical resection in properly selected patients. Although second lung resections, especially ipsilateral ones, are associated with high morbidity, the majority of these are categorized as mild, and with thorough patient selection, second resections can be performed safely for the patient.
Authors’ Contributions- -
José Soro-García: Acquisition, collection and analysis of data, drafting the article, critical revision and approval of the final version.
- -
Ángel Cilleruelo-Ramos: Acquisition, collection and analysis of data, drafting the article, critical revision and approval of the final version.
- -
Álvaro Fuentes-Martín: Analyzed the data, read and approved the manuscript.
- -
Mauricio Alfredo Loucel Bellino: Analyzed the data and critical revision and approval of the final version.
- -
David Alfonso Mora Puentes: Critical revision and approval of the final version.
- -
Génesis Isabel Victoriano Soriano: Critical revision and approval of the final version.
- -
José María Matilla González: Conceived and designed the study, analyzed the data, read and approved the manuscript.
None declared.
Conflicts of InterestThe authors state that they have no conflict of interests.