CURRENT SITUATION AND LONG-TERM CONSEQUENCES OF COVID-19 INFECTION
More infoTwo dizzying years have passed since the 2020 arrival of the SARS-CoV-2 pandemic causing more than 500 million infections and 6 million deaths. Physicians treating hospitalized COVID-19 patients have gone from using antivirals with in vitro activity but that subsequently failed to demonstrate efficacy in clinical trials (e.g., hydroxychloroquine, lopinavir-ritonavir and ivermectin) and anti-inflammatories without the initial certainty of their possible benefit, to organizing ourselves through large investigator-driven platforms (e.g., RECOVERY, SOLIDARITY, ACTIV-2, and ACTIV-3/TICO) or company-based randomized clinical trials (RCT) allowing us to optimize the treatment of COVID-19 in three large areas: antivirals (including monoclonal neutralizing antibodies [mAbs]), anti-inflammatories, and anticoagulants (Fig. 1).
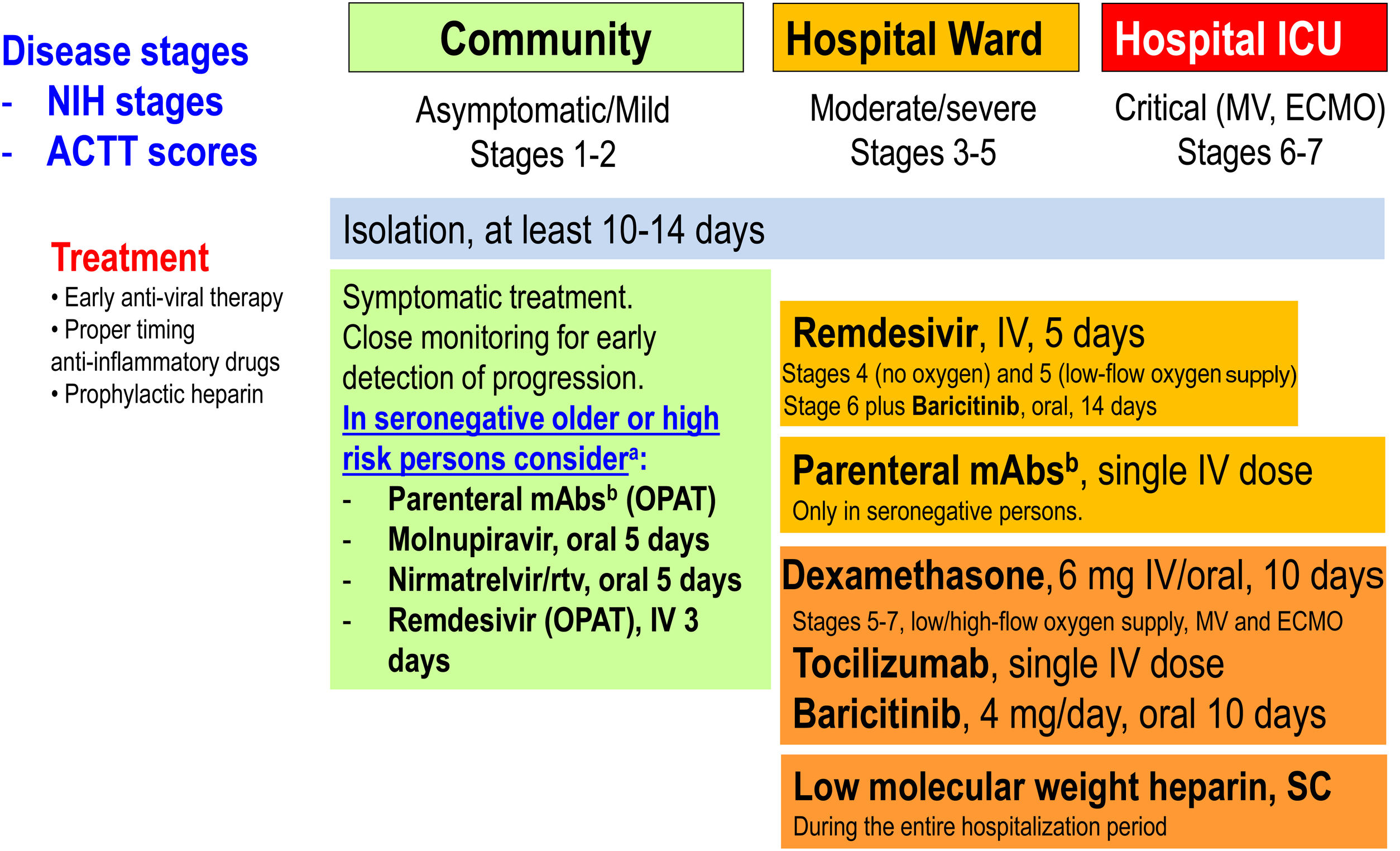
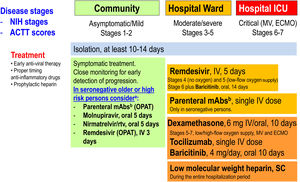
Therapeutic management of COVID-19 (April 2022). ACTT scores on the ordinal scale are as follows: 1, not hospitalized, no limitations of activities; 2, not hospitalized, limitation of activities, home oxygen requirement, or both; 3, hospitalized, not requiring supplemental oxygen and no longer requiring ongoing medical care (used if hospitalization was extended for infection control reasons); 4, hospitalized, not requiring supplemental oxygen but requiring ongoing medical care (COVID-19-related or other medical conditions); 5, hospitalized, requiring any supplemental oxygen; 6, hospitalized, requiring non-invasive ventilation or use of high-flow oxygen devices; 7, hospitalized, receiving invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO); and 8, death. NIH stages. Asymptomatic or pre-symptomatic infection: Individuals who test positive for SARS-CoV-2 using a virologic test (i.e., a nucleic acid amplification test or an antigen test), but who have no symptoms that are consistent with COVID-19; Mild illness: Individuals who have any of the various signs and symptoms of COVID-19 (e.g., fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, loss of taste and smell) but who do not have shortness of breath, dyspnea, or abnormal chest imaging; Moderate illness: Individuals who show evidence of lower respiratory disease during clinical assessment or imaging and who have oxygen saturation (SpO2)≥94% in room air at sea level; Severe illness: Individuals who have SpO2<94% in room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2)<300mmHg, respiratory frequency >30 breaths per minute, or lung infiltrates >50%; Critical illness: Individuals who have respiratory failure, septic shock, and/or multiple organ dysfunction. ECMO: extracorporeal membrane oxygenation; ICU: intensive care unit; IV: intravenous; mAbs: monoclonal neutralizing antibodies; MV: mechanical ventilation; OPAT: outpatient parenteral antimicrobial therapy; rtv: ritonavir; SC: subcutaneous. (Modified from Ambrosioni et al. Lancet HIV. 2021;8:e294–305.)aNo data in previously infected or vaccinated patients. bCheck variant of concern (VoC) for antiviral activity.
Remdesivir, a nucleotide prodrug that inhibits viral RNA polymerase, was the only antiviral receiving FDA and EMA approvals in May and June 2020, respectively, for treating hospitalized patients with moderate, severe or critical COVID-19. Recently, however, there has been a change of paradigm in SARS-CoV-2 antiviral treatment with the aim of very early treatment for outpatients (first 5–7 days from symptom onset) with mild to moderate COVID-19 but with risk factors for progression, to avoid hospital admission and death. In so doing, we have three antivirals with different profiles that will determine its clinical use: intravenous (IV) remdesivir and two new oral antivirals: molnupiravir and nirmatrelvir-ritonavir. The PINETREE placebo-controlled RCT,1 with 562 non-hospitalized patients, allocated IV remdesivir (200mg loading dose followed by 100mg/day during 2 days) or a placebo, showing an 87% reduction in hospitalization and death (0.7% vs. 5.3%, respectively). Molnupiravir also inhibits SARS-CoV-2 replication by inducing RNA mutagenesis. Molnupiravir was ineffective in treating hospitalized patients with COVID-19 but, in an RCT with 1433 non-hospitalized adults (MOVE-Out),2 it showed a 30% reduction in hospitalization and death compared with the placebo group (6.8% vs. 9.7%, respectively). Molnupiravir (800mg) or a placebo were given orally twice daily for 5 days (40 pills in total). The final results were lower than those observed in the interim analysis (50% reduction in hospitalization and death) for unclear reasons. A similar open-label RCT in India included 1218 patients treated with molnupiravir and was presented in February 2022 to the last CROI meeting (Kumarasamy et al. Oral-09). It showed a 65% reduction in hospitalization and death compared with the standard of care (1.5% vs. 4.3%, respectively). In addition, there were some theoretical concerns that molnupiravir may cause mutations in human DNA, although the FDA concluded the risk of genotoxicity is very low (pregnancy is a contraindication), or hasten the development of new viral variants, something not shown so far. Nirmatrelvir inhibits the main SARS-CoV-2 protease, which cleaves viral polyproteins into non-structural proteins essential for replication, and is co-formulated with ritonavir to inhibit CYP3A metabolism of nirmatrelvir and achieve therapeutic levels. An RCT (EPIC-HR) including 2246 non-hospitalized participants with COVID-19 randomly assigned either nirmatrelvir-ritonavir orally thrice daily for 5 days (30 pills in total) or a placebo.3 The results showed an 87% reduction in hospitalization and death compared with the placebo group (0.8% vs. 6.3%, respectively). The main limitation of this protease inhibitor is being boosted with ritonavir, a strong CYP3A4 inhibitor with important drug-drug interactions, meaning many medications should not be co-administered or should be temporarily stopped. In December 2021, the FDA approved the emergency use authorization (EUA) of both drugs. This notwithstanding, it is very important to highlight that these studies have been carried out in unvaccinated people and against variants of concern other than Omicron, although in vitro studies have not shown any loss of efficacy against Omicron.4 Moreover, immunocompromised individuals were rarely represented in RCTs and their effect in such populations remains largely unknown.
Anti-SARS-CoV-2 monoclonal antibodies (mAbs) may be used as pre-exposure prophylaxis (PrEP) to prevent symptomatic SARS-CoV-2 infection in highly exposed individuals, as post-exposure prophylaxis (PEP), as early treatment in outpatients with mild to moderate COVID-19 and high risk of progression to prevent hospital admission and death, and to treat hospitalized individuals with severe COVID-19.5 mAbs bind to specific regions of the SARS-CoV-2 spike protein. They neutralize virus particles in vitro and in vivo, thus preventing new cell infections and halting virus replication. Most mAbs developed to date were identified from subjects who recovered from SARS-CoV-2 infections early in the pandemic. Omicron spike mutations in their binding sites greatly reduce their binding affinity and antiviral efficacy in vitro.6 Sotrovimab®, as an exception, was identified from a patient who recovered from a SARS-CoV-1 infection. It binds to a spike motif conserved in all sarbecoviruses and variants, including Omicron BA.1. In vitro studies, however, suggest Omicron's BA.2 subvariant might be more resistant to sotrovimab than BA.1. The tixagevimab-cilgavimab combination (Evusheld®) also shows shifts in fold-change against BA.1 and less to BA.2, but increased concentrations of the drug result in 100% virus inhibition. Omicron has become fully resistant to the REGN-CoV2 (casirivimab and imdevimab) cocktail, among other mAbs. Bebtelovimab has received EUA by the FDA because it is fully able to neutralize Omicron.
In the PrEP field, Evusheld® (and previously bamlanivimab-etesevimab) showed in SARS-CoV-2 highly exposed subjects that it was able to reduce symptomatic COVID-19 infections by more than 50%.7 The long half-life and anti-Omicron activity of Evusheld® make it a suitable drug to prevent COVID-19 in subjects unable to produce endogenous neutralizing antibodies due to immune suppression or in those not tolerating SARS-CoV-2 vaccines. When initiated early in the course of COVID-19 (first 5–7 days from symptom onset) in high-risk subjects infected with non-Omicron variants of concern in the outpatient settings, they are able to reduce the risk of hospitalization by 50–80%.8 However, as in the antiviral RCTs in non-hospitalized patients, these studies included neither fully vaccinated individuals nor subjects infected with the Omicron variant. Finally, the only mAb combination with proven clinical benefit in hospitalized patients is the REGN-CoV2 cocktail, which improved survival when added to the standard of care only in seronegative subjects in the RECOVERY RCT.9 The potential harm of mAbs in hospitalized seropositive patients required further studies.
The administration of corticosteroids was controversial or contraindicated at the beginning of the pandemic. However, the RECOVERY RCT10 demonstrated giving dexamethasone (6mg/day) during 10 days versus placebo decreased mortality in patients with COVID-19 requiring oxygen therapy, with or without mechanical ventilation, becoming the cornerstone of anti-inflammatory treatment and the first drug reducing mortality in patients with severe COVID-19. A subsequent meta-analysis from the World Health Organization (WHO)11 confirmed these findings. Despite evidence supporting the benefits of corticosteroids, retrospective/prospective studies have described a lack thereof in some patient subgroups such as patients aged >80 years.12 Another study in France13 including ICU-admitted patients found elevated mortality in patients aged <60 years without any increase in inflammation markers.
The second anti-inflammatory drug is tocilizumab, an anti-interlukine-6 blocker. In a RCT from the RECOVERY group,14 it decreased in-hospital mortality and other outcomes in hospitalized patients with respiratory failure. However, the sensitivity analyzed showed benefits neither in patients >80 years, in women, in patients with more than 7 days of symptoms nor in patients needing mechanical ventilation. Interestingly, the effect of concomitant administration of corticosteroids increased survival by 12%, and therefore in clinical practice dexamethasone plus a single dose of tocilizumab is part of the standard of care. In addition, baricitinib (a JAK inhibitor) in patients hospitalized for COVID-19 significantly reduced the risk of death, but the size of benefit was somewhat smaller than suggested by previous trials.15 The total randomized evidence to date suggests that JAK inhibitors (chiefly baricitinib) reduce mortality in patients hospitalized for COVID-19 by about one fifth.
Finally, the use of colchicine has conflicting results, and preliminary experiences with oral fluvoxamine or inhaled budenoside have shown encouraging preliminary results.
Many questions still need to be answered to optimize COVID-19 treatment in different scenarios and patient phenotypes, but now, 2 years later, the medical community is ready to answer them.
FundingJMM received a personal 80:20 research grant from Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain, during 2017–2023.
Conflict of interestsJMM has received consulting honoraria and/or research grants from AbbVie, Angelini, Contrafect, Cubist, Genentech, Gilead Sciences, Jansen, Lysovant, Medtronic, MSD, Novartis, Pfizer, and ViiV Healthcare. AT has received honoraria or has been in advisory boards from MSD, Pfizer, Janssen, Synaergen, and Jubavis. RP has received consulting honoraria and/or research grants from Gilead Sciences, MSD, Theratechnologies, Lilly, and ViiV Healthcare. All conflicts outside the submitted work.