Multi-drug resistant tuberculosis (MDR-TB), defined as resistance to at least rifampicin and isoniazid, has become one of the major obstacles to global TB control.1 Until very recently, the MDR-TB treatment required multiple drugs (some of questionable efficacy) for a minimum of 18 months, with multiple adverse reactions and cure rates not exceeding 60%.1–5 However, the arrival of new drugs with action against Mycobacterium tuberculosis, much more bactericidal and sterilizing,2–5 has completely changed this landscape. Therefore, the Spanish Society of Pneumology and Thoracic Surgery (SEPAR) already advised, in its 2020 recommendations, that these cases of MDR-TB could be treated with a 6-month regimen of bedaquiline+levofloxacin+linezolid.2 However, it wasn’t until December 2022 that World Health Organization recommended (these) the shortened 6-month regimens for MDR-TB treatment, though with a combination of 4 drugs (bedaquiline+pretomanid+linezolid+moxifloxacin).3
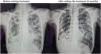
We present the case of a 37-year-old woman, regular smoker, originally from Russia, who had recently traveled to Italy and stayed there for several months. She reported a 6-mont-clinic of persistent cough, purulent sputum, minimal exertion dyspnea, hyporexia, and a weight loss of 20kg. On physical examination, she exhibited only a low-grade fever and severe malnutrition (height 175cm and 48kg). Laboratory tests revealed anemia with a hemoglobin level of 9.4g/dL, leukocytosis of 23,700/mm3 with lymphopenia (1020/mm3, 4.3%), and thrombocytosis of 1,282,000/mm3. Chest radiography showed extensive involvement of the lung parenchyma with bilateral micronodular pattern, cavities in both hemithorax (largest measuring 13 and 7cm in right lung) and multiple mediastinal lymph nodes. Microbiological testing revealed positive smear (auramine staining) and PCR for Mycobacterium tuberculosis, with rifampicin resistance detected by GeneXpert MTB/RIF. Sensitivity molecular and phenotypic testing confirmed resistance to isoniazid (mutations in katG and inhA genes), ethambutol, and aminoglycosides, but without fluorquinolones resistance.
With the confirmation of MDR-TB, treatment was initiated with levofloxacin 1000mg/day, linezolid 600mg/day, and bedaquiline (400mg daily for two weeks and followed by 200mg three days/week for 6 months). The patient received supervised home treatment for the scheduled six months, with good compliance, significant clinical improvement, and negative smear and mycobacterial cultures at the 2nd, 5th, and 6th months of treatment, leading to acceptance of cure of the disease. The patient did not experience relevant side effects during treatment. Fig. 1 shows radiological improvement at the end of treatment compared to the beginning. One year after completing treatment, the patient remained cured with no signs of potential relapse in subsequent visits (clinically asymptomatic and negative mycobacterial culture every two months in the follow-up).
The present case supports that with a shortened 6-month treatment and only 3 drugs, with good bactericidal and sterilizing activity,4,5 these forms of MDR-TB can be cured, even in cases with cavitary and bilateral involvement, as recommended by SEPAR in 2020.2 This approach could further streamline the treatment of these complex MDR-TB patients compared to the latest recommendations by the WHO.3
We believe it is important that these evidences are known, which can help to greatly simplify the management of these complex MDR-TB cases, especially in Spain and other countries where is very difficult to achieve pretomanid. Anyway, we support putting pressure on all levels to make pretomanid easy to obtain, so that it can be used in these MDR/pre-XDR-TB patients.
Conflict of interestsThe authors state that they have no conflict of interests.