Lung cancer (LC) is usually diagnosed at advanced stages with only a 12% 5-year survival. Trials as NLST and NELSON show a mortality decrease, which justifies implementation of lung cancer screening in risk population. Our objective was to show survival results of the largest LC screening program in Spain with low dosage computed tomography (LDCT).
MethodsClinical records from International Early Lung Cancer Detection Program (IELCAP) at Valencia, Spain were analyzed. This program recruited volunteers, ever-smokers aged 40–80 years, since 2008. Results are compared to those from other similar sizeable programs.
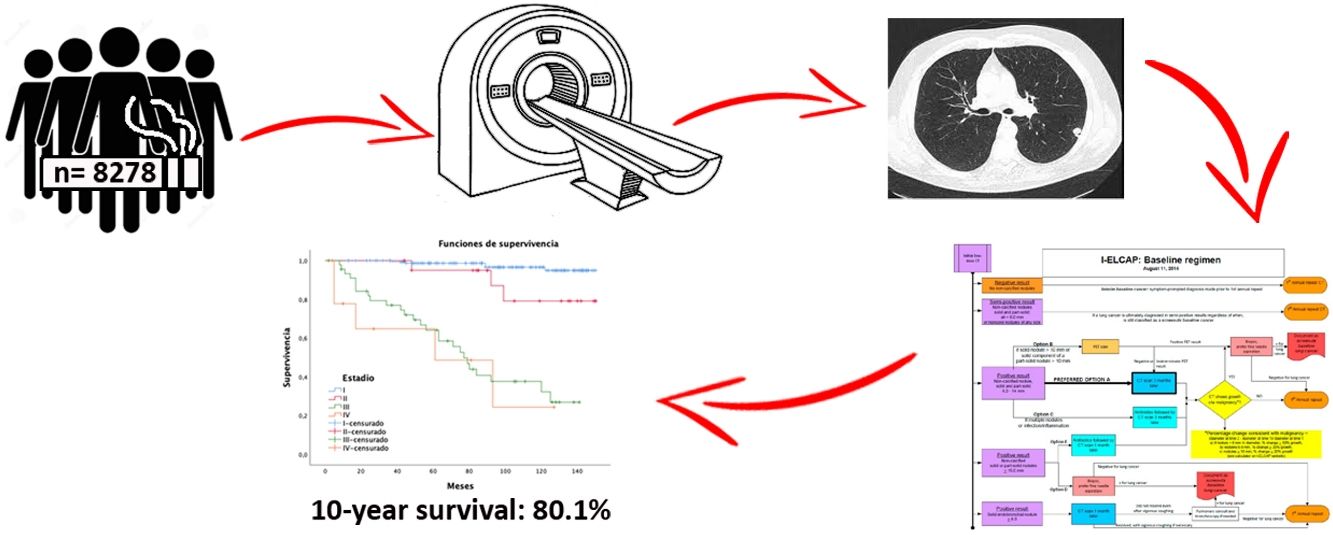
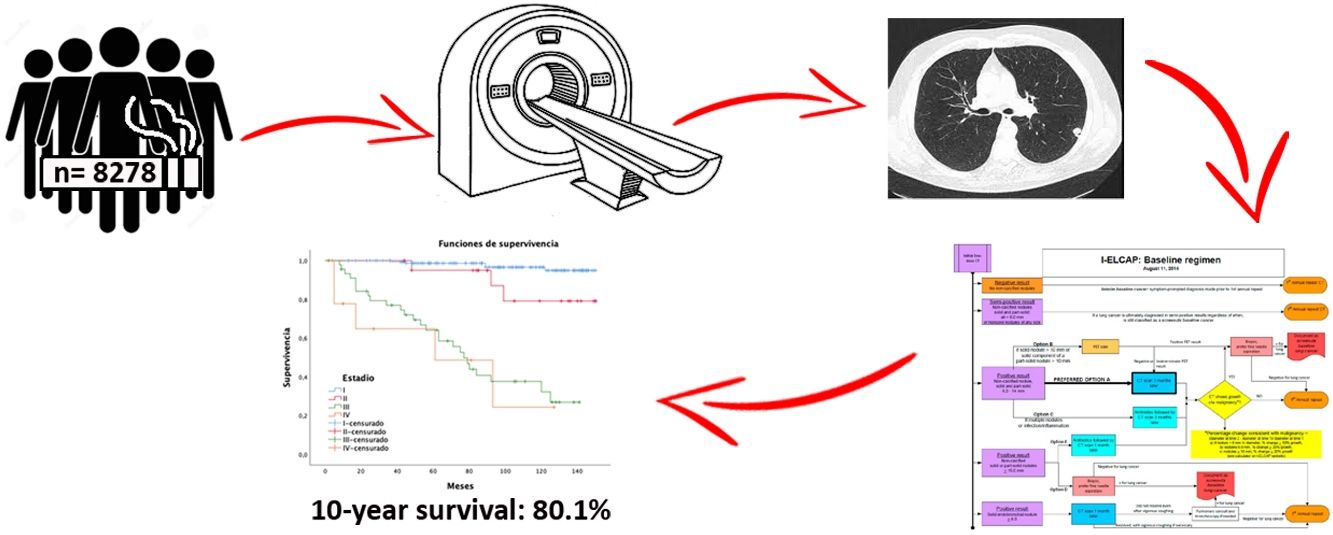
ResultsA total of 8278 participants were screened with at least two-rounds until November 2020. A mean of 6 annual screening rounds were performed. We detected 239 tumors along 12-year follow-up. Adenocarcinoma was the most common histology, being 61.3% at stage I. The lung cancer prevalence and incidence proportion was 1.5% and 1.4%, respectively with an annual detection rate of 0.17. One-year survival and 10-year survival were 90% and 80.1%, respectively. Adherence was 96.84%.
ConclusionLargest lung cancer screening in Spain shows that survival is improved when is performed in multidisciplinary team experienced in management of LC, and is comparable to similar screening programs.
El cáncer de pulmón (CP) se diagnostica habitualmente en estadios avanzados con una supervivencia media a cinco años del 12%. Ensayos como el National Lung Screening Trial (NLST) y el NEderlands Leuvens longkanker Screenings ONderzoek (NELSON) demuestran una reducción de la mortalidad que justifican la implantación del cribado en población de riesgo. Nuestro objetivo es presentar los resultados de supervivencia del programa de cribado de CP más amplio de España con tomografía computarizada de baja dosis (TCBD).
MétodosSe analizaron los datos del programa Internacional de Detección Precoz de CP (IELCAP) en Valencia, España. Este programa reclutó fumadores o exfumadores con una edad entre 40-80 años. Se comparan los resultados con otros programas de similar tamaño.
ResultadosUn total de 8.278 participantes fueron reclutados con al menos dos rondas de seguimiento, hasta noviembre de 2020 (62,8% varones), realizando una media de seis rondas de cribado por individuo. Diagnosticamos 239 tumores en 12 años de seguimiento. El adenocarcinoma fue el tumor más frecuente con un 61,3% en estadio I. Las tasas de prevalencia e incidencia fueron de 1,5% y 1,4%, respectivamente, con una tasa de detección anual de 0,17. Las tasas de supervivencia cáncer específica a cinco años fueron del 90 y del 80,1% a 10 años. La adherencia fue de 96,84%.
ConclusiónLa experiencia del programa más amplio de España demuestra que la supervivencia se mejora cuando se realiza en equipos multidisciplinares con experiencia en CP y es similar a programas similares.
Lung cancer (LC) is the leading cause of cancer death: prevalence is low, but diagnosis is usually made in advanced stages,1,2 conferring a very low overall 5-year survival rate (6%–18%).2 In Europe, about 400,000 LC cases are diagnosed, 300,000 deaths occur annually, and the 5-year average survival rate is 12%.3 In Spain, LC is the third most common tumor,4,5 and about 29,000 new cases are diagnosed every year. Smoking is the most important etiopathogenic factor (70% of cases), so campaigns to prevent people from starting and to help smokers quit, together with the introduction of early detection measures, can reduce LC morbidity rates.6
The main objective of cancer screening programs is to diagnose cancer in early stages when it is still curable.7 Several publications have shown that 85% of cases of early-stage LC can be detected with low-dose computed tomography (LDCT), and the 10-year survival rate in stage I disease is as high as 88%.8–10
Several LDCT screening programs have demonstrated their usefulness in at-risk populations. The National Lung Screening Trial (NLST) showed a 20% reduction in mortality compared to chest X-ray screening after a mean follow-up of 6.5 years, and this approach now has a grade B recommendation from the US Preventive Services Task Force (USPSTF) in smokers of ≥20 pack-years aged 50–80 years or former smokers of <15 pack-years.11
In 2015, the European Respiratory Society (ERS) and the European Society of Radiology (ESR) recommended screening for LC with LDCT12 in comprehensive, quality-assured programs within a clinical trial or in a care setting in medical centers with multidisciplinary teams that meet minimum requirements. Initiatives calling for screening have emerged in Spain, but this strategy is not included in the national cancer program of the Ministry of Health.13
In Europe, several projects are underway,14,15 such as the Multicenter Italian Lung Detection (MILD) and NEderlands-Leuvens Longkanker Screenings ONderzoek (NELSON) randomized trials. These studies use less restrictive inclusion criteria than NLST, and have reported a 24% reduction in LC mortality in men, and an even greater reduction (33%–59%) in women,16 while 50% of cancers detected with LDCT were stage I, compared with 75% at advanced stages in the control group. However, the European Network of Health Technology Assessment Agencies (EUnetHTA) does not recommend this strategy.17
Our main objective is to analyze the survival and mortality of LC diagnosed by screening with LDCT in Spain's largest screening program. The secondary objective was to describe the characteristics of our study population and diagnosed LCs.
MethodsBetween June 2008 and November 2020, taking advantage of an opportunistic screening unit based in our institution, we contacted possible candidates by telephone and recruited 8546 asymptomatic volunteers to our study. Inclusion criteria from 2008 to 2016 were ≥50 years, smoker ≥10 pack-years. After 2016 we included smokers ≥15 pack-years, and former smokers <15 years smoke-free, with no personal history of cancer, except for basal cell carcinoma of the skin. Informed consent approved by the Ethics Committee of our hospital was collected from all participants.
After epidemiological data and risk factors were collected, 8278 volunteers were selected. They performed a baseline LDCT and at least one more for annual monitoring. Negative cases continued with a third annual follow-up scan and subsequently every 18 months until 72 months. The International Early Lung Cancer Action Program (I-ELCAP)18 was used for diagnosis and follow-up of findings (see www.ielcap.org/protocols), with a retrospective analysis of the data collected in this cohort.
Between 2008 and 2016, the baseline study was considered positive if solid or partially solid non-calcified nodules (NCN) ≥5mm or non-solid nodules ≥8mm were observed. Subsequently, nodules ≥6mm were considered positive, in line with changes made in the I-ELCAP protocol. If positive, patients were followed up with LDCT at 3 months, positron emission tomography-computed tomography (PET-CT) or biopsy, according to size or criteria highly suggestive of malignancy. If infection was suspected, antibiotic treatment and monthly LDCT follow-up were recommended. If a partial or complete resolution was demonstrated, the next follow-up was performed after 1 year.
Pre-existing tumors were defined as nodules detected in the baseline study and emerging tumors were those that appeared de novo during LDCT follow-up and were diagnosed as cancer. Findings during screening prompting a diagnostic procedure, such as biopsy, surgery, bronchoscopy, or PET, that yielded a benign result were considered false positives.
Chronic obstructive pulmonary disease (COPD) was classified by spirometry as mild (forced expiratory volume in 1 second [FEV1] ≥80% predicted), moderate (50%≥FEV1<80%), severe (30%≥FEV1<50%), and very severe (FEV1<30%), according to Global Initiative for Chronic Obstructive Lung Disease criteria.19 Patients diagnosed with LC were stratified according to the 8th TNM international classification,20 with an expected follow-up of 10 years. Emphysema was graded following the CT imaging criteria defined by the I-ELCAP protocol.18
Data on LC mortality were collected from the patient's medical history and the causes of overall mortality from the electronic medical record updated in November 2020 – overall mortality being defined as deaths from LC plus deaths from other causes such as cancers other than LC, vascular accidents, sepsis, etc. The overall mortality rate was calculated by dividing all deaths by the sum of disease-free months.
Statistical analysisFor statistical analysis, we used the R program (version 3.6.3) and RStudio (version 1.2.5033) as well as the SPSS Statistics version 26 (IBM Corp., Armonk, NY), with a p value of <0.05 being considered significant. For continuous variables with normal distribution, we calculated the mean, median and range, and variables without normal distribution were analyzed using a Wilcoxon signed-rank test. For categorical variables, we collected the relative frequency distribution and used the contingency table analysis with Fisher's test or χ2. Calculations of pre-existing and emerging tumors were performed according to the diagnostic round.
For risk calculations, we performed a bivariate logistic regression that included the variables age, sex, family history, body mass index (BMI), pack-years, COPD, and emphysema.
Using mortality data, we described 5- and 10-year survival of LC patients using Kaplan–Meier curves and we used the Cox method of proportional risks to calculate risk of death using the variables age, sex, surgical treatment, and stages.
ResultsStudy patient characteristics are shown in Table 1. Of the 8546 volunteers included, 8278 underwent at least 2 LDCTs, except those who were diagnosed with LC in the baseline study who had only 1 follow-up test (Table 2). In total, 62.8% were men and 37.2% were women. The overall adherence rate was 96.84%, with a median follow-up of 72 months (mean=67.6, standard deviation [SD]=27.03). The mean number of LDCTs performed during the study period was 6 (1–15) per person, 15.4% of whom had emphysema in the baseline LDCT. FEV1/forced vital capacity (FVC) for the assessment of airway obstruction was only collected in 3387 individuals.
Characteristics of the selected population with 2 or more LDCT follow-ups.
| Characteristics | Women (n=3089) | Men (n=5189) | Total (n=8278) | LC-women (n=67) | LC-men (n=172) |
|---|---|---|---|---|---|
| Age | 55 (52–59) | 57 (53–61) | 56 (53–61) | 58 (55–62) | 61 (57–66) |
| <50 years | 3 | 9 | 12 | 0 | 0 |
| 50–55 years | 1586 | 1954 | 3540 | 16 | 17 |
| 55–60 years | 863 | 1733 | 2596 | 23 | 46 |
| 60–65 years | 444 | 897 | 1341 | 19 | 53 |
| 65–70 years | 157 | 407 | 564 | 7 | 44 |
| >70 years | 35 | 189 | 224 | 2 | 12 |
| BMI | 24.9 (22.5–29.9) | 27.60 (25.4–30) | 26.70 (24.2–29.4) | 24 (21.7–26.4) | 27.4 (24.3–29.8) |
| Pack-years | 26.5 (17–36) | 33 (21–47) | 30.6 (19–42) | 34 (20.4–47.4) | 48 (38.9–69.3) |
| <15 p-y | 585 (18.9%) | 642 (12.4%) | 1227 (14.8%) | 7 | 4 |
| 15–30 p-y | 1184 (38.3%) | 1539 (29.7%) | 2723 (32.8%) | 20 | 23 |
| ≥30 p-y | 1319 (42.7%) | 3008 (57.9%) | 4327 (52.3%) | 40 | 145 |
| Smoking status | |||||
| Smoker | 1943 (63%) | 2679 (51.6%) | 4622 (55.8%) | 42 | 117 |
| Former smoker | 1142 (37%) | 2513 (48.4%) | 3655 (44.2%) | 25 | 55 |
| Months of follow-up | 72 (44–85) | 73 (49–90) | 72 (48–87.5) | 89 (66–126.5) | 92.5 (52–130.1) |
| LDCT follow-ups | 6 (4–7) | 6 (5–7) | 6 (5–7) | 4 (2–6) | 4 (3–6) |
| Family history of LC | 707 (8.5%) | 978 (11.8%) | 1685 (20.4%) | 21 (31.3%) | 41 (23.8%) |
| Emphysema in baseline LDCT | p<0.0001 | p=0.071 | |||
| No | 2690 | 4280 | 6970 | 45 | 100 |
| Minimal | 307 | 672 | 979 | 20 | 46 |
| Moderate | 67 | 185 | 252 | 2 | 21 |
| Severe | 4 | 31 | 35 | 0 | 5 |
| COPD | p=0.0084 | p=0.76 | |||
| Normal | 325 | 438 | 763 | 1 | 3 |
| Mild | 950 | 1465 | 2415 | 11 | 33 |
| Moderate | 58 | 137 | 195 | 53 | 134 |
| Severe | 2 | 12 | 14 | 2 | 2 |
Median and interquartile range or percentage are shown in parentheses.
COPD: chronic obstructive pulmonary disease; LC: lung cancer; LDCT: low-dose computed tomography; p-y: pack-years.
Number of LDCTs per study participant.
| No. of LDCTs | Number of participants | % |
|---|---|---|
| 1 | 8278 | 16.61 |
| 2 | 8237 | 16.53 |
| 3 | 7819 | 15.69 |
| 4 | 7223 | 14.49 |
| 5 | 6255 | 12.55 |
| 6 | 5225 | 10.48 |
| 7 | 3294 | 6.61 |
| 8 | 1896 | 3.80 |
| 9 | 931 | 1.87 |
| 10 | 401 | 0.80 |
| 11 | 176 | 0.35 |
| 12 | 76 | 0.15 |
| 13 | 25 | 0.05 |
| 14 | 7 | 0.01 |
| 15 | 3 | 0.01 |
%: Corresponds to the total percentage of LDCTs performed.
LDCT: low-dose computed tomography.
A total of 239 tumors were diagnosed in 223 people, with 2 patients presenting 3 tumors and 12 presenting 2 tumors. In all, 124 tumors were pre-existing (ratio by sex=1.5%), while 115 were emerging (ratio by sex=1.4%); no significant differences in diagnosis by sex were detected for either category (p=0.131). Among patients with multiple tumors, 12 were metachronous and 3 were synchronous; of these, one refused surgery and the other 2 were not treated surgically.
In total, 113 patients (1.35%) were false positives, whether at baseline or in the follow-up LDCTs, determined as follows: 43 punctures, not performed in 10 cases due to resolution of the lung lesion or refusal by the patient; 10 bronchoscopies; 37 PETs; and 23 surgical biopsies with a benign result.
Cases diagnosed as LC comprised significantly more smokers (>13 pack-years) than the rest of the study population (43.75 vs. 30, p<0.001), older patients (59 vs. 56 years, p<0.001), and patients with more severe COPD (p=0.021), while there were no differences in BMI (26.55 vs. 26.70, p=0.1919). Only the variables age (p<0.0001), pack-years (p<0.0001), and BMI (p<0.001) were statistically significant in a logistic regression analysis.
The predominant histological type was adenocarcinoma (75.3%), of which 21 cases were minimally invasive and 10 in situ, followed by squamous (11.3%) and neuroendocrine disease (11.3%), of which 8 were carcinoid tumors and 18 were small-cell neuroendocrine tumors. A total of 76.6% were early stage (I and II), while 4.2% were in stage IV, as shown in Table 3. The annual rate of emerging disease was 0.17 and the number of participants needed to detect an LC in one year was 416.
Incidence, stages, and histology of diagnosed and treated LCs.
| Total | Pre-existing on LDCT | Emerging on LDCT | ||
|---|---|---|---|---|
| Incidence of LDCT | 239 | 124 | 115 | |
| LC rate/100,000 | 433.6 | nc | nc | |
| Incidence individual/year | ||||
| LC stage | χ2=58,911p=0.117Fisher (p=0.1214) | |||
| I | 161 (67.4%) | 76 (61.3%) | 85 (73.9%) | |
| II | 22 (9.2%) | 13 (10.5%) | 9 (7.8%) | |
| III | 46 (19.2%) | 27 (21.8%) | 19 (16.5%) | |
| IV | 10 (4.2%) | 8 (6.5%) | 2 (1.7%) | |
| Surgery | p=0.4575 | |||
| Non-surgical | 44 (18.5%) | 25 (10.5%) | 19 (8%) | |
| Segmentectomy/lobectomy | 188 (79.2%) | 94 (39.5%) | 96 (39.9%) | |
| Pneumectomy | 3 (1.3%) | 3 (1.3%) | 0 | |
| Refused surgery | 2 (0.8%) | 1 (0.4%) | 1 (0.4%) | |
| Histology | p=0.076 | |||
| Adenocarcinoma | 180 (75.3%) | 100 (41.8%) | 80 (33.5%) | |
| Adenosquamous | 1 (0.4%) | 1 (0.4%) | 0 | |
| Squamous | 27 (11.3%) | 8 (3.3%) | 19 (7.9%) | |
| Large-cell | 2 (0.8%) | 2 (0.8%) | 0 | |
| Small-cell neuroendocrine | 18 (7.5%) | 6 (2.5%) | 12 (5.0%) | |
| Carcinoid | 8 (3.4%) | 7 (2.9%) | 1 (0.4%) | |
| NOSa | 2 (0.8) | 0 | 2 (0.8%) | |
| Death due to LC | 37 (16.6%) | 25 (11.2%) | 12 (5.38%) | |
| LC mortality rate (per 1000) | 0.77 | 0.52 | 0.25 | |
LC: lung cancer; LDCT: low-dose computed tomography; nc: not computed.
The total percentage of deaths was 1.4%, of which 0.5% were deaths from LC and 0.9% from other causes. Our overall mortality rate was 1.99 per 1000. The specific LC mortality rate was 16.6% (37 people), of which 4 were in stage I (1.83%), 3 in stage II (1.38%), 25 in stage III (1.46%), and 5 in stage IV (2.29%). Most deaths were due to disease progression (34 cases) or respiratory complications (3 cases).
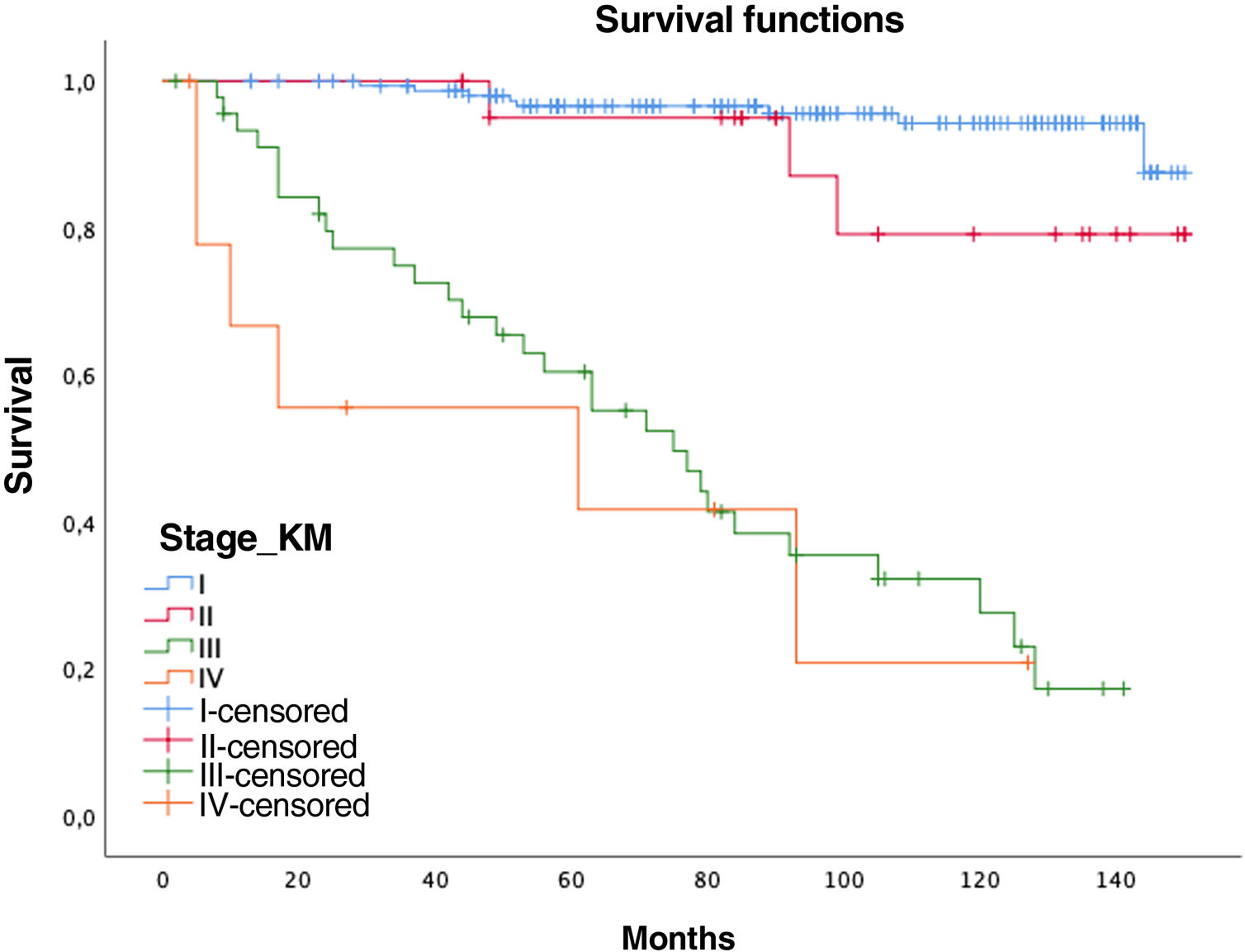
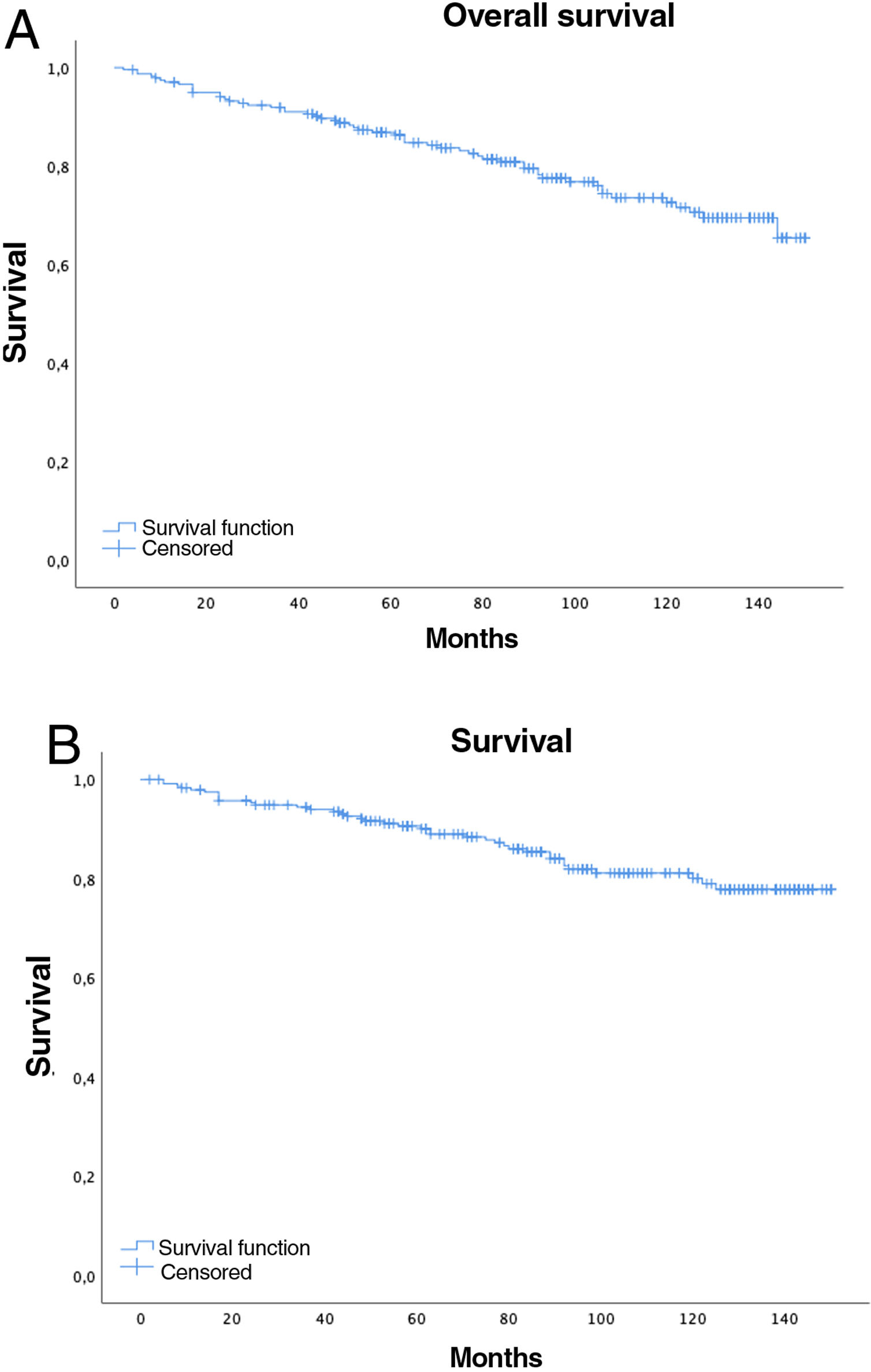
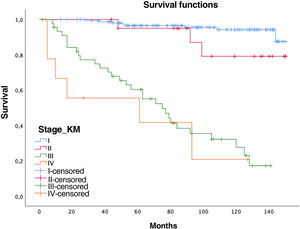
Women had a lower relative risk (RR) of death at 5 years (RR women=0.73 vs. RR men=1.37), but the differences were not significant (p=0.1711, confidence interval [CI]=0.47–1.15). In contrast, the variables age (RR=0.95; CI=0.95–0.96), pack-years (RR=1.01, CI=1.01–1.02), and surgical treatment (RR=0.75; CI=0.57–0.99) showed statistical significance (p<0.05). In this series, 44 patients with tumors did not undergo surgery (12 stages I–II and 32 stages III–IV), with 5- and 10-year survival rates of 70.7% and 40.5%, significantly lower than tumors that were operated on (94% and 87.9%, respectively) (Fig. 1).
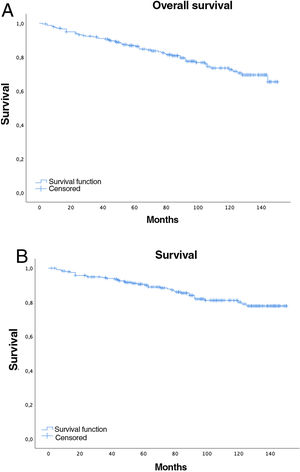
Five-year overall survival was 86% (CI: 81.4–90.8) and 10-year overall survival was 72.2% (CI: 65.6–79.4), with a median of 91 months (mean 89; range=2–150, CI: 83.64–94.75), while for stages I–II median survival was 98.5 months and 61 months for stages III–IV (Fig. 2A).
Overall 5-year LC survival (Fig. 2B) was 90% (CI: 86–94.1) and 10-year LC survival was 80.1% (CI: 74.1–86.5), while 5- and 10-year survival for stage I disease was 98.5% (CI: 96.6–100) and 97.4% (CI: 94.5–100), respectively.
DiscussionOur results are comparable with published evidence in terms of pre-existing and emerging LC, histology, and staging. In the baseline round, the rate of pre-existing LC in our series was 1.5%, similar to ITALUNG, and slightly higher than NELSON (0.9%) and the Pamplona International Early Lung Cancer Detection Program (P-IELCAP) (1%),21 but lower than the UK Lung Cancer Screening Trial (UKLS) (2.1%). This article reports the experience of the largest LDCT screening program in LC with the second-longest follow-up in Spain.
In 2000, the Clínica Universitaria de Navarra joined the I-ELCAP, and reported a yield similar to other European programs, with a rate of stage I LC diagnoses of 85%.21 In June 2008, our hospital also joined the project, reporting 52.08% stage I tumors, a preliminary 5-year overall survival of 58.5% and specific LC survival of 67.1%, with a rate of 75.8% for surgical cases.22 The proportion of emerging tumors in our series was 1.4%, the same as in P-IELCAP and similar to NLST (1.5%)21 (Table 4). Our 10-year LC survival rate for stage I disease was 97%. This improved outcome may be explained by the greater number of controls, since the inclusion criteria and diagnostic algorithms are very similar,18 with surgical resecability rates similar to other series.23 Our low false positive rate of 1.36% is comparable only to the 1.2% reported by the NELSON study, which used stricter volumetric criteria.16 All other programs show higher rates, ranging from 5.2% in the Danish Lung Cancer Screening Trial (DLCST) to 24.2% in the NLST.24
European screening studies and NLST.
| Project inclusion | Age and follow-ups | Follow-up (years) | % Cancer in baseline study | % Cancer in follow-up studies | LC mortality | Overall mortality |
|---|---|---|---|---|---|---|
| NELSON15,422 | 50–75 years4 follow-ups: baseline, 1–2, and 2.5 years | 10 years | 0.9 | 0.9 | Women: 0.61 (0.35–1.06)Men: 0.74 (0.60–0.91) | Not published |
| DLCST4104 | 50–70 yearsAnnual (5 years) | 10 years | 0.8 | 0.7 | 1.03 (0.66–1.60) | 1.01 (0.82–1.25) |
| MILD4099 | 49–75 yearsAnnual (10 follow-ups) or biennial (5 follow-ups) | 10 years | Yearly: 1Biennial: 0.5 | Yearly: 0.5Biennial: 1 | 0.73 (0.47–1.112) | 0.94 (0.73–1.20) |
| LUSI4052 | 50–69 yearsAnnual (5 years) | 8.8 years | 1.1 | 0.5 | 0.72 (0.45–1.116)Women: 0.31 (0.1–0.94)Men: 0.92 (0.54–1.58) | 0.98 (0.79–1.22)Women: 0.82 (0.47–1.42)Men: 1.02 (0.80–1.29) |
| DANTE1264 | 60–74 yearsAnnual, 5 follow-ups | 8.35 years | 2.3 | 5.85 | 0.54 (0.41–0.7) | 1.65 (1.42–1.91) |
| NLST5.3454 | 55–74 yAnnual (3 follow-ups) | 7.4 years | 1 | 0.85 (0.75–0.96) | 0.94 (0.88–1.00) | |
| P-IELCAP2989 | ≥40 y.Annual (3 follow-ups) | 3.5 years | 1 | 1.4 | na | na |
na: not available.
Adenocarcinoma was the most frequent histological type, with no sex differences, similar to other screening programs.16,25 Overall, 61.3% of our LC cases were stage I, lower than P-IELCAP (85%) and the Lung Cancer Screening Intervention (LUSI) (82%), but similar to NELSON (64%) and slightly better than NLST, which reported a total of 39.6% stage I cases,26 and MILD, which had a total of 50% in stage I.25
Our 10-year survival rate for stage I disease of 97.5% is higher than that of P-IELCAP (80%) and I-ELCAP (88%). This is probably due to the low rate of minimally invasive adenocarcinomas (8.8%), adenocarcinoma in situ (4.2%), and carcinoid tumors (3.3%) in our series. The rate of advanced stage disease was low, with 8% of emerging tumors and 2% of those pre-existing tumors in stage IV.
This paper underlines the significance of both COPD and emphysema detected on CT as risk factors for LC. These importance of these parameters in the selection of volunteers has already been demonstrated in the literature,21 since the greater the severity of centrilobular emphysema, the greater the risk of LC.27 The same premise applies to the pack-year index.28
Age is the independent variable taken into account in all screening programs. Inclusion criteria range between 50 and 80 years, since beyond this limit the risk of comorbidities due to overdiagnosis and inefficiency increases. In our series, we included people aged 50–80 years (with the exception of 12 individuals under 50 years) with the highest inclusion peak occurring in the 50 to 60-year range (74.12%). The peak range for LC was from 60 to 65 years, very similar to that of other publications.21
The specific LC mortality rate at 12 years of follow-up was 16.6%, slightly lower than in NELSON,16 possibly because our screening program had a longer overall follow-up combined with shorter visit intervals.
Our population showed a high adherence rate of 96.8%, higher than NELSON (90%), and significantly higher than the 56% adherence rate reported by P-IELCAP. This lower rate in the P-IELCAP study was probably due to different health coverage conditions: under an agreement with the Spanish National Health Service, the services offered by our Foundation are fully subsidized. Improving adherence is a multifactorial problem requiring multiple strategies, including the contribution of the nursing staff.29
Evidence has shown that female sex is gaining more specific weight, with an increase in mortality rates of 6.5% in the last 5 years.30 In our series, women accounted for 37.32% of the overall cohort and 28.03% of LC diagnoses; specific mortality among individuals diagnosed with LC was 19.4%, with no significant differences from men (p=0.46).
Our study has certain limitations. Firstly, it is a single-center screening trial with a single study group that is not completely comparable to the aforementioned randomized trials. Secondly, we recruited younger participants with a lower smoking history threshold than those used in other studies. Generally, screening programs base their selection on smoking habit, with some uniformity in the pack-year index, with variations of >15 pack-years in NELSON16 and >30 pack-years in NLST.26 Our study, that began in 2008 with limited evidence, included smokers <15 pack-years, but in combination with other conditions, such as family history of LC, occupational exposure, previous respiratory diseases, genetic predisposition or socioeconomic status, since these factors significantly increase the risk of LC.3,27,29
Thirdly, we cannot rule out the existence of selection bias and, in particular, a healthy volunteer effect. This was described in P-IELCAP,21 in which the mildest COPD cases were the ones with the best adherence.
In conclusion, our results are comparable with published evidence in terms of pre-existing and emerging LC, histology, and stage. LC-specific survival and mortality is improved with LDCT screening when performed by multidisciplinary teams with experience in the diagnosis and management of LC.
FundingThis paper has not received any funding.
Conflict of interestsThe authors state that they have no conflict of interests.