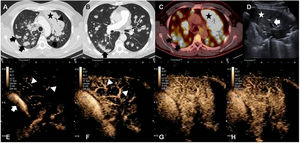
We report the case of a 58-year-old man with Langerhans cell histiocytosis with skin and lung involvement, diagnosed by biopsy. Lung biopsy was performed by thoracoscopy with atypical resection of the right lower lobe. After 3 years of follow-up, a chest X-ray revealed opacity in the left apex and multiple bilateral nodules. Computed tomography (CT) and fluorodeoxyglucose (FDG) positron emission tomography (PET-CT) were performed, showing extensive lung involvement (see Fig. 1) manifesting as diffuse consolidation in the left upper lobe (LUL) and multiple pulmonary nodules, primarily with peribronchovascular distribution. Ultrasound pre- and post-intravenous contrast administration was also performed to guide the biopsy needle and obtain tissue for histological study. Images showed nodules and subpleural consolidations, along with extensive opacity in the LUL with air bronchogram (see Fig. 1). After the administration of ultrasound contrast in the LUL, late uptake (by the bronchial arteries) and early elimination were observed, suggesting malignant involvement.1 Two percutaneous core needle biopsies of 2 different lesions were performed under ultrasound guidance. Pathology diagnosis of the biopsies revealed Langerhans cell sarcoma (LCS). After 3 treatment lines, the patient progressed and died 9 months after diagnosis.
(A) Chest CT scan in lung window. Extensive consolidation in the left upper lobe (star) and multiple bilateral pulmonary nodules (arrows). (B) Chest CT scan in lung window showing suture material from atypical resection for biopsy (arrow). (C) PET-CT: mediastinal window. Increased FDG uptake in left upper lobe (star) and in multiple pulmonary nodules (black arrows). (D) Ultrasound: consolidation in left upper lobe (star) and air bronchogram (arrow) are shown. (E–H) Contrast-enhanced ultrasound. (E, F) Contrast uptake in pulmonary artery (arrow) and its branches (arrowhead). No parenchymal uptake is observed. (F) Delayed uptake in pulmonary parenchyma, indicating irrigation of the mass from the bronchial arteries.
LCS is a very rare disease with a poor prognosis. Pulmonary presentation is exceptional among the reported cases. Most of the cases described in the literature are limited to a single organ, most frequently the skin and the lymph nodes. Other affected organs may include the spleen, bone marrow, liver, kidney, thymus and lung.2
The immunophenotypic profile of LCS shows CD1a, vimentin, CD21, CD35, CD68, CD56 and S100 protein expression.2 CT can identify both focal and disseminated involvement that correlates on PET-CT with intense FDG uptake in the affected area.3 Contrast-enhanced ultrasound highlights neoplastic parenchyma and differentiates it from pulmonary atelectasis and the rest of the parenchyma, optimizing sample collection yield in image-guided biopsy.4
In the literature, we found 2 reports of cases of LCS located primarily in the lung. Both cases were initially interpreted as lung carcinomas but were eventually diagnosed as LCS by immunohistochemistry.5
Conflict of interestsThe authors state that they have no conflict of interests.