During the first COVID-19 peak and after administering it to approximately 500 patients in Wuhan,1 the Chinese health authorities included tocilizumab (TCZ), an interleukin 6 receptor antagonist, for the treatment of severe SARS-CoV-2 pneumonia.
With increasing evidence of its effectiveness in severe COVID-19,2 in Spain the Ministry of Health authorizes TCZ expanded access, prioritizing the inclusion of patients in clinical trials.3,4 As it is an immunosuppressive agent, it is advisable to screen for latent infections caused by intracellular bacteria, parasites and viruses, such as M. tuberculosis, which could be reactivated during treatment with TCZ5 in patients who may be candidates to receive it for long periods. Additionally, a recent, non-peer-reviewed report indicates that, like pandemic influenza, SARS CoV-2 might increase the number of tuberculosis (TB) cases and related mortality.6 Moreover, new cases of coinfection TB-COVID-19 have been drescribed.7 Finally, several original works, reviewed during the first European congress on SARS CoV-2 (ECCVID) held online between September 23 and 25, 2020, coincide in stating that mortality is influenced by SARS CoV-2, as much in patients with a history of TB as in those with active TB.8
From March 15 to May 15 2020, an IGRA test, Quantiferon TB Gold Plus (QFN-Qiagen, Venlo, The Netherlands), was requested9 in our hospital for patients with SARS CoV-2 confirmed by PCR who met clinical (on the COVID-19 severity scales), radiological (new onset or progression of the initial pulmonary infiltrates) and biological (IL-6>40pg/ml) criteria for treatment with TCZ in order to evaluate their immune response to latent tuberculosis infection (LTBI) before starting TCZ. Additional blood tests were done to examine other immune parameters, including CD4+ and CD8+ lymphocyte counts. Patients gave their verbal consent before undergoing treatment and for the use of their clinical data and storage of surplus samples in a biological bank (biobank) for research purposes. Both approval of the Hospital Pharmacy Committee and authorization from the Research Ethics Committee were obtained before treatment began.
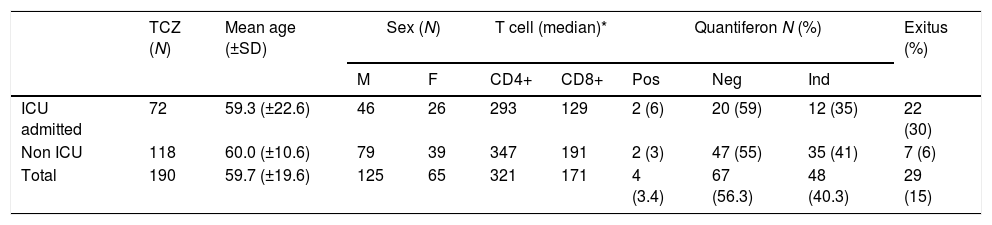
Among the 190 patients treated with TCZ, the mean age (±SD) was 59.7(19.6) years and 125 (71%) were male. Seventy-two (38%) required ventilation in Intensive Care (ICU). Twenty-two (30% of those requiring ventilation) and 7 (6% of the non ICU patients) died as a result of refractory distress (ARDS). Valid samples for QFN were obtained in 119 patients (63%). The results were negative in 67 (56.3%), indeterminate in 48 (40.3%) and positive in 4 (3.4%). Upon retesting the patients with indeterminate results after 8 weeks, all but one who tested positive had negative results. The CD4+ and CD8+ counts extracted prior to TCZ administration showed a median of 321cells/mL (IQR: 49–1356) and 171 (IQR: 16–1083), respectively. There were no differences in these T-lymphocyte counts between patients admitted and not admitted to the ICU (Table 1).
Demographic data and results of Quantiferon and CD4+/CD8+ counts in patients receiving TCZ.
| TCZ (N) | Mean age (±SD) | Sex (N) | T cell (median)* | Quantiferon N (%) | Exitus (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | F | CD4+ | CD8+ | Pos | Neg | Ind | ||||
| ICU admitted | 72 | 59.3 (±22.6) | 46 | 26 | 293 | 129 | 2 (6) | 20 (59) | 12 (35) | 22 (30) |
| Non ICU | 118 | 60.0 (±10.6) | 79 | 39 | 347 | 191 | 2 (3) | 47 (55) | 35 (41) | 7 (6) |
| Total | 190 | 59.7 (±19.6) | 125 | 65 | 321 | 171 | 4 (3.4) | 67 (56.3) | 48 (40.3) | 29 (15) |
Glossary: ICU=Intensive Care Unit; N=cases; M=male; F=female; Pos=Positive; Neg=Negative; Ind=Indeterminate. *P value non significant between ICU and non ICU (>0.05).
These data illustrate that SARS CoV-2, while producing an exacerbated inflammatory response, may be associated with T-lymphocyte depletion-dysfunction, which may reduce the capability of Quantiferon TB Gold Plus to identify the LTBI response in patients with moderate and severe COVID- 19. In our cohort, severe COVID-19 patients with cytokine release syndrome, showed medians of CD4+ and CD8+ below 350 and 200cells/mL, respectively, which were probably the cause of the higher-than-expected indeterminate QFN values (40.3%). Similar results have been seen in several IGRA-based LTBI studies in immunosuppressed individuals.10,11
Since SARS CoV-2 could influence the dynamics of M. tuberculosis, specific follow-up of recovered COVID-19 patients at high risk factors of developing active TB should be considered independently of the results of QFN.
Itziar Arrieta, Joan Gómez-Junyent, Silvia Gómez-Zorrilla, Alicia González, Ana Guelar, Roberto Güerri, Elisabeth Lerma, Emili Letang, Inmaculada López-Montesinos, María Milagro Montero, Nuria Prim, Helena Sendra, Ana Siverio, Luisa Sorlí, Judit Villar and Juan Pablo Horcajada.
Please see a list of the members of the COVID-19 Infectious Disease Team Hospital del Mar in Appendix A.