Influenza vaccines have been in the public health system in Galicia (northwest Spain) since 1991, with the elderly, health workers and people at higher risk of influenza complications being the target population of the seasonal vaccination campaign.1 Given the expected intense influenza activity after two previous mild seasons during the pandemic and based on the successful experience of other countries,2 Galician public health authorities decided to also include children aged from 6 to 59 months in the 2022–2023 influenza vaccination campaign.
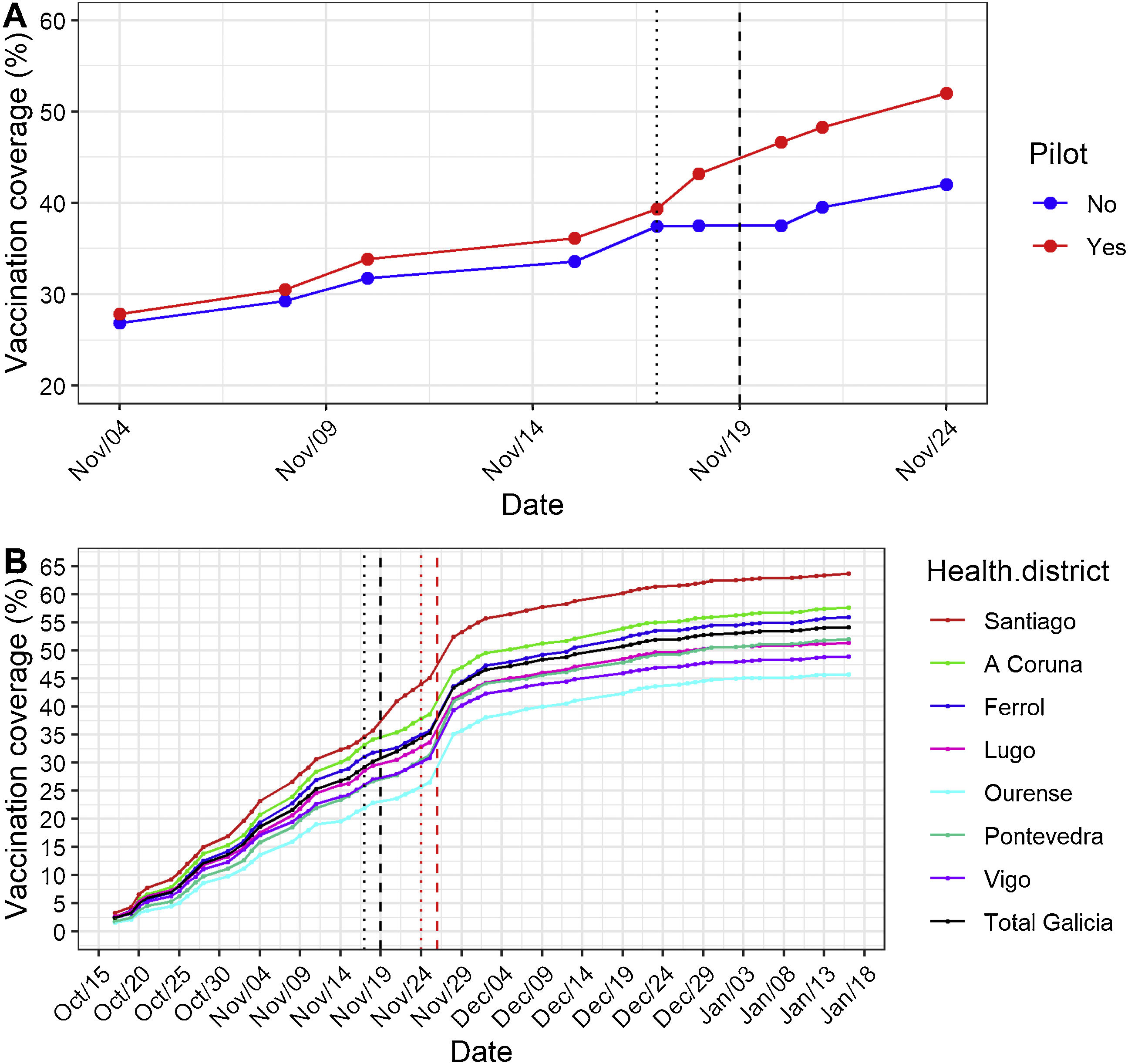
The influenza vaccination campaign for children was launched on October 17th, 2022, two weeks earlier than originally planned, as influenza activity had already started in the region by the end of September.3 The campaign was officially announced, pediatricians were informed, and parents received an informative short messaging service (SMS). Vaccines were delivered through the regular vaccination points in primary care, targeting a total of 69,142 children and aiming for 60% coverage. By November 8th, 23% of the target children had already received the first vaccine dose. Despite the good pace of vaccination, given the growing activity of influenza and the increased demand in primary care centers, the Galician public health authorities decided to implement a pilot study within the region to assess if vaccine uptake could be boosted. This pilot (Pilot 1) targeted 3293 families with unvaccinated children between 6 and 59 months of age within a 20-minute isochrone of the Santiago de Compostela hospital. These families received a flexible appointment for vaccination by SMS with a QR code that enabled them to get their children vaccinated at any time in the Hospital Clínico Universitario de Santiago facilities during the weekend of November 19–20th in addition to their usual vaccination points. A similar strategy had already been successfully used for COVID-19 vaccination, providing easy parking and access to the outpatient facilities of the hospital, where a multidisciplinary clinic with 6 simultaneous vaccination points was set. The rationale behind this pilot was to further engage families in influenza vaccination by facilitating access to the vaccination point during the weekend, mitigating worktime/schooltime clashes, and kindly reminding them to get their children voluntarily vaccinated using the usual vaccination points or the hospital during the weekend. The Pilot 1 SMS was sent on November 17th. The intervention had the immediate effect of increasing vaccination coverage by 2.5% compared to the areas not included in the pilot (Fig. 1A). During the vaccination weekend at the hospital, a further 3.5% extra coverage was achieved, resulting in an overall increase of 6%. A multivariate linear model adjusted for days, and health care centers estimated an increase of 5.26 (95% CI: 4.02, 6.5) percent in vaccination coverage for the Pilot 1.
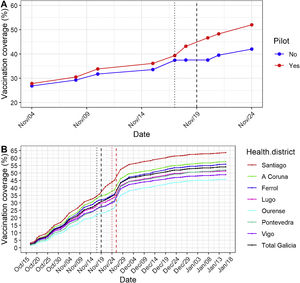
(A) Influenza vaccination coverage in children aged 6–59 months in Galicia. Effect of Pilot 1 plan in influenza vaccination coverage comparing the target population with the rest of the region (blue). Electronic appointments sent by SMS (black-dotted line) and vaccination weekend (black-dashed line). (B) Effect of Pilot 1 (black) and Pilot 2 (red) in influenza vaccination coverage in total, and in each healthcare district. Electronic appointments sent by SMS (dotted line) and vaccination weekend (dashed line).
The Galician public health system is organized in seven healthcare districts that provide universal access to all the population.4 Following Pilot 1, the health authorities decided to implement a second pilot study (Pilot 2) that was extended to the seven Galician healthcare districts. A total of 43,900 families with unvaccinated children between 6 and 59 months received the SMS with the QR code and the invitation to bring their child for influenza vaccination to the nearest hospital among any of the 14 hospitals in the region, in addition to the usual vaccination points, during the weekend of November 26th and 27th. Pilot 2 also had a significant impact, with a 5% average increase in vaccine coverage across all healthcare districts (Fig. 1B). The overall effect of Pilots 1 and 2 was analyzed using a linear model to estimate their effect on vaccination coverage. The model was adjusted for healthcare districts and the day after the initiation of the vaccine campaign. Pilot 1 showed a significant 5.84% increase (95% CI: 2.75, 8.92) of vaccination coverage in the overall healthcare district (Santiago de Compostela district including pilot 1 control healthcare centers) compared to the other 6 healthcare districts (A Coruña, Ferrol, Lugo, Ourense, Pontevedra, Vigo) and to the average coverage in all the region (Galicia) (Fig. 1B). Pilot 2 showed a significant 4.48% (95% CI: 3.17,5.78) increase in vaccine coverage compared to the average coverage prior to the Pilot 2 intervention. The vaccination campaign ended in January 15th 2023 with a 54.97 coverage in Galicia (Fig. 1B).
Galicia region in Spain has consistently led vaccination uptake against COVID-19 in all age groups and at all vaccination stages within Spain.5 Leveraging on this experience, Galician authorities have successfully boosted the influenza vaccine uptake in children during the 2022 influenza vaccination campaign by increasing access to vaccination through additional mass vaccination sites with long opening hours over the weekend, and flexible electronic appointments. Mass vaccination sites have been an essential component of the strategy to reach a sufficient level of community immunization rapidly, efficiently, and equitably during COVID-19 pandemic.6 In addition, global information campaigns in the media and reiterative personalized messaging to the parents of the eligible children have proved to be of help for this goal.7 The early start of influenza activity this season, the co-circulation with other viruses such as SARS-CoV-2 and respiratory syncytial virus, and the uncertainty of their consequences made the acceleration of the influenza vaccination campaign particularly important. Several factors can slow down the pace of vaccine coverage,8 and two key ones are distance to the vaccination center and access to vaccination, as was also shown with COVID vaccination weekend walk-in center.
Primary care and community clinics have typically been the main centers for influenza vaccine administration, but mass vaccination sites can be essential in achieving the necessary levels of community vaccination in a short period of time.9 Site selection based on accessibility and space, community engagement, operational flow, trained staff, and adequate medical oversight, proper signaling, and electronic recording of vaccine administration are key factors to be considered.10,11 Our double pilot experience shows a consistent 5% average increase in influenza vaccination coverage – reaching 10% in the area where the pilot was repeated – which highlights the interest and success of this strategy. Personal telephone messages informing the parent of the eligible children and providing an electronic citation for vaccination, information and outreach campaigns, increased accessibility through flexible timetables including the weekend, and electronic registration and live follow-up of the vaccination campaign are essential tools for this strategy. Additionally, these types of initiatives can be planned in a relatively brief time, facilitating their implementation according to the epidemiological situation and the actual vaccination coverage.
The success of vaccines relies on actual vaccine uptake. Influenza vaccine acceptance is typically low as compared to other routine vaccines. Boosting influenza vaccine uptake in children leveraging on past lessons learnt during COVID-19 vaccine campaigns may be useful for other countries as part of their measures promoting influenza vaccination in children.
AuthorshipAll authors should have made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
FundingResearch activities are supported by grants from Instituto de Salud Carlos III (ISCIII): ReSVinext (PI16/01569/Cofinanciado FEDER, F.M.T.), Enterogen (PI19/01090/Cofinanciado FEDER, F.M.T.), OMI-COVI-VAC (PI PI22/00406/Cofinanciado FEDER,F.M.T.) and consorcio Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CB21/06/00103); GEN-COVID (IN845D 2020/23, F.M.T.) and Grupos de Referencia Competitiva (IIN607A2021/05, F.M.T.).
Conflict of interestsFederico Martinón-Torres has received honoraria from GSK, Pfizer Inc, Moderna, AstraZeneca, Sanofi Pasteur, MSD, Seqirus, Biofabri and Janssen for taking part in advisory boards and expert meetings and for acting as a speaker in congresses outside the scope of the submitted work. Federico Martinón-Torres has also acted as principal investigator in randomized controlled trials of the above-mentioned companies as well as Ablynx, Gilead, Regeneron, Roche, Abbott, Novavax, and MedImmune, with honoraria paid to his institution.