Osteoporosis is a systemic skeletal disease characterized by low bone mass and/or microarchitectural deterioration of bone. Osteoporosis is a risk factor for fractures specially in patients with advanced COPD. The aim of this cross-sectional study was to determine the frequency of osteoporosis and vertebral fracture in COPD patients.
MethodsWe evaluated 91 COPD patients and compared to 82 healthy subjects (control group) matched for gender and age. We measured the bone mineral density by means of dual energy X-ray absorptiometry (DXA), evaluating the lumbar vertebra (L1–L4), proximal femur and total femur and evaluated vertebral fractures in thoracic and lumbar spine using X-ray. We questioned patients whether they had had any fall that resulted in any fracture in the past five years.
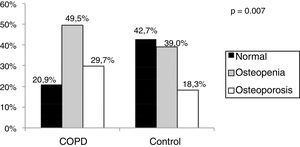
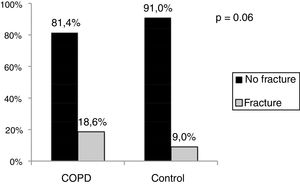
ResultsMales comprised 60.4% of COPD group and 57.3% of the control group. Mean age was 66.2±9.2 years for COPD group and 64.2±8.8 for the control group. The frequency of osteoporosis in the COPD group was 29.7% and 18.3% in control group (p=0.007). The frequency of vertebral fractures was 18.6% in the COPD group and 9.0% in control group (p=0.06). The frequency of reported falls at resulting in any fracture was 36.3% in the COPD group and 7.3% in control group (p=0.001).
ConclusionsOur data indicate that COPD patients present a high frequency of osteoporosis and falls seem to be an important factor for vertebral fracture.
La osteoporosis es una enfermedad sistémica esquelética caracterizada por una baja densidad ósea y/o un deterioro de la microarquitectura del hueso. Constituye un factor de riesgo de fracturas, especialmente en pacientes con EPOC avanzada. El objetivo de este estudio transversal fue determinar la incidencia de osteoporosis y de fracturas vertebrales en pacientes con EPOC.
MétodosSe evaluaron 91 pacientes con EPOC y se compararon con 82 sujetos sanos (grupo control) emparejados por sexo y edad. Se midió la densidad mineral ósea mediante densitometría ósea (DXA) de la vértebra lumbar (L1-L4), el fémur proximal y el fémur total. Las fracturas en vértebras torácicas y lumbares se evaluaron mediante rayos X. Se preguntó a los pacientes por posibles caídas en los últimos cinco años que pudieran haber resultado en fractura.
ResultadosLos hombres representaron el 60,4% del grupo EPOC y el 57,3% del grupo control. La edad media fue 66,2±9,2 años en el grupo EPOC, y 64,2±8,8 años en el grupo control. La incidencia de osteoporosis en el grupo EPOC fue del 29,7%, y del 18,3% en el grupo control (p=0,06). Se registró una incidencia de caídas resultantes en fracturas del 36,3% en el grupo EPOC y del 7,3% en el grupo control (p=0,001).
ConclusiónNuestros datos sugieren que los pacientes con EPOC presentan una mayor incidencia de osteoporosis. Las caídas parecen ser un factor relevante en la fractura vertebral.
Chronic obstructive pulmonary disease (COPD) is characterized by a chronic airflow limitation that is generally progressive.1,2 This disease has become one of the main public health problems worldwide, and the number of persons afflicted continues to grow. According to GOLD,3 COPD will be the fifth cause of incapacity and the third cause of death worldwide during the first half of the XXI century.
Currently COPD is considered as a disease with systemic repercussions and recent data point to the fact that it is associated to osteoporosis.4 Osteoporosis is characterized by low bone mineral density and alterations in microarchitecture, increasing the risk of fractures, imposing lower mobility and leading to changes in postural balance. The World Health Organization defines osteoporosis as a bone density ≥2.5 standard deviations below the bone density of a normal young adult.5
Previous studies report an osteoporosis prevalence between 24 and 44% in the population with COPD in several countries around the world.4,6 The etiology for bone mass loss in COPD is unknown; it is probably multifactorial, including female sex, corticosteroid therapy, hypogonadism, smoking, lack of physical conditioning, vitamin D deficiency and chronic inflammation.4,5
Progressive bone mass loss leads to osteoporosis and poses a high risk for vertebral or femur fracture. They cause a significant increase in morbidity and mortality and worsens the respiratory function and mobility.7,8 The early recognition of bone mass reduction in the COPD population is important, as it would allow the establishment of a preventive and therapeutic measures that potentially could minimize the probability of falls and fractures. The real frequency of fractures related to osteoporosis is not known in patients with COPD, as the existing results tend to be discrepant.9–13 A study that evaluated only men with COPD or asthma found that these patients has a two-fold risk of having vertebral fracture compared to individuals without pulmonary disease9; another study that also evaluated only men with COPD observed that 63.3% of the patients that used corticosteroids systemically presented with vertebral fracture10; a study with patients from both genders with a GOLD II to IV classification showed the frequency of fractures ranging between 9 and 69%, depending on the severity and use of corticosteroids.11 The only study that assessed a control group as well, however with only 34 patients with moderate and severe COPD, observed a frequency of 59% in spine fractures in patients with COPD.12 In a study of COPD patients with varying degrees of severity and including both genders, the prevalence of fractures was of 36.5%, notwithstanding this, these patients were not compared to a control group.13
The objective of this study was to assess the frequency of osteoporosis and spine fractures in patients with COPD and compare them to a control group. As far as we know, our study is the first to assess the frequency of spine fractures in patients with COPD of both sexes and all degrees of severity of COPD with a paired control group for gender and age.
Materials and methodsPatient population and study designA cross-sectional study was carried out in 173 individuals. Of these, 91 were COPD patients followed-up at the Pulmonary Rehabilitation Center of Escola Paulista de Medicina/Federal University of São Paulo and 82 were healthy individuals (control group), from both genders and ages higher than 40 years.
The study was approved by the Institutional Review Board (number 1738/10) and all patients signed an informed consent.
Clinical characteristicsCOPD patients were included and classified according to the GOLD spirometric classification (I–IV), with ages over 40 years old and clinically stable during the previous three months. The inclusion criteria in the control group included individuals with ages higher than 40, non-smoking or former smokers (that had stopped smoking for more than one year), and that had no pulmonary disease. The control group was recruited from University employees, close relatives of the COPD patients and from the community. Individuals with other chronic diseases such as cancer or renal failure were excluded; and those unable to answer the questionnaires and undergo spirometry. Physical activity was defined as regular physical exercise practice more than twice per week. The patients and control group were asked according to the IPAC questionnaire, an international questionnaire for physical activity and, despite its limitation, is widely used in large trials.
The medical history included data on diets, alcohol and cigarette consumption, levels of physical activity, medication being used and the patients were questioned about having had a fracture or fall in the last 5 years. In the physical exam, stature and weight were recorded for a subsequent calculation of the body mass index (BMI) and for Dual Energy X-Ray Absorptiometry (DXA).
SpirometryTo carry out the spirometry, patients were oriented not to make use of any bronchodilating medication, as well as coffee or tea in the last twelve hours before the exam. Volume was checked daily with a 3l-syringes. Spirometry was done with a portable device (ndd, Easy One, Switzerland) and the forced vital capacity (FVC), forced expiratory volume in the first second (FEV1) and the FEV1/FVC ratio were measured according to the American Thoracic Society recommendations.14 The maneuver was carried out repeatedly until three curves were recorded; 400mcg of salbutamol were administered and a new spirometry was repeated 15min after. Reference values were taken from Nhanes III.15
Dual energy X-ray absorptiometry – DXAThe procedure involving the DXA was carried out at the Osteometabolic Outpatient Center at the Endocrinology Division. Exams followed the positioning protocols and were always done using the same device (Lunar-GE, model NT), using the same technique and analyzed by the same expert in osteometabolic diseases. Assessment was done in fasting, during the morning period, with the patient in supine position, with an average duration of 45min per exam. We measured the bone mineral density by means of dual energy X-ray absorptiometry (DXA), evaluating the lumbar vertebra (L1–L4), proximal femur and total femur. The bone mass density (BMD) measured correlates to the peak bone mass and is expressed as a T-score which is the number of standard deviations below or above peak bone mass for the relevant gender. T-score values between −1.0 and −2.5 are definable for osteopenia and T-scores below −2.5 are definable for osteoporosis, in at least one of the three regions of interest.16
Vertebral radiogramRadiograms (XRay) of the thoracic and lumbar spine were done in the antero-posterior planes (AP) and profile (P), to view and carry out a detailed evaluation of the vertebral plateaus. All X-rays were analyzed by the same thoracic radiologist specialist. Gennant semi-quantitative method, excluding grade I.17
Daily calcium consumptionTo quantify each patient's daily calcium consumption, 300mg of calcium were considered for each glass of milk or yoghurt, and 200mg of calcium for each slice of cheese ingested.18 The calcium consumption ingested daily was also classified regarding the normal daily values, according to WHO recommendations, 1000mg/day for adults.19
Statistical analysisTo calculate the sample, the Silva et al.20 article was used as a basis, in which the osteoporosis prevalence in patients with COPD is 42% and in control group is 15%.21 The mean difference of 27% was divided by the standard deviation, resulting in the expected effect (E/S) of 0.46. With an α=0.05 and a β of 80%, it was necessary to have 85 individuals per group to have statistical power in the study.
The numerical variables were described as averages and standard deviation (SD). The category variables were expressed in absolute numbers and percentages of the total. The Pearson Correlation was used to verify the association between the variables analyzed. The Chi-square test was used to compare proportions or ratios with dichotomic variables. The multivariate logistic analysis was used to evaluate the possible risk factors that could influence the development of osteoporosis and fractures (COPD, personal history of fracture, alcohol consumption, oral corticosteroid use, and history of rheumatoid arthritis, age, sex, BMD, and current smoking).
ResultsInitially, 199 individuals were assessed, but three patients from the COPD group were excluded as the spirometry did not fit in the spirometric COPD diagnosis, six for used osteoporosis medication and 17 from the control group, six for abnormal spirometry, four due to withdrawal of the informed consent, three for not being able to accomplish the spirometry, three for used osteoporosis medication and one due to renal failure. Finally, 173 individuals ended up being included in the study, 91 of them with COPD and 82 as the control group (CG). In both groups, the greatest percentage was of men, however without statistical difference. The group with COPD presented moderate pulmonary obstruction with FEV1 post-BD of 55±21% and the CG fell within normal values (Table 1).
Demographic characteristics and lung function.
| COPD (n=91) | Control (n=82) | p | |
|---|---|---|---|
| Sex, n (%) | 0.68 | ||
| Male | 55 (60.4) | 47 (57.3) | |
| Female | 36 (39.6) | 35 (42.7) | |
| Age (years) | 66.2±9.2* | 64.2±8.8 | 0.08 |
| BMI (kg/m2) | 27.3±5.2 | 29.4±4.3 | 0.006 |
| Spirometry (post BD) | |||
| FEV1 (L) | 1.30±0.61 | 2.53±0.65 | 0.001 |
| FEV1 (%) | 55.1±21.4 | 100.8±15.5 | 0.001 |
| FVC (L) | 2.77±0.87 | 3.24±0.81 | 0.001 |
| FVC (%) | 80.7±18.3 | 98.3±16.1 | 0.001 |
| FEV1/FVC | 0.50±0.12 | 0.80±0.06 | 0.001 |
| Smoking, n (%) | 0.001 | ||
| Never smoker | 12 (13.2) | 56 (68.3) | |
| Former smoker | 75 (82.4) | 26 (31.7) | |
| Current smoker | 4 (4.4) | 0 (0) | |
| Pack-years | 50.65±28.3 | 17.50±11.0 | 0.001 |
| DM | 16 (17.6) | 20 (24.7) | 0.25 |
| HAS | 52 (57.1) | 41 (50.6) | 0.39 |
| Cariopatia | 10 (11) | 3 (3.7) | 0.07 |
| Dislipidemia | 13 (14.3) | 9 (11.1) | 0.53 |
The COPD Group presented higher frequency of osteoporosis and osteopenia (p=0.007) than the control group (Fig. 1).
The COPD group presented a higher frequency of vertebral fractures than the control group (Fig. 2), but no statistical difference.
Patients with COPD presented a higher frequency (36.3%) of falls leading to fractures than the control group (7.3%) (p<0.001) and a lower regarding the daily calcium consumption (791.2±358.2) in COPD group than the control group (905.5±379.2) (p=0.04). There was no difference between the groups of physical activity, 40% in COPD and 50% in control group (p=0.22).
When we evaluated clinical and demographic characteristics only for the COPD group regarding bone mass, we observed that isolatedly the group with osteoporosis was older, had a lower BMI and had more severe disease, but a multivariate logistic regression analysis including the three above factors plus gender showed that age was the only risk factor for osteoporosis and osteopenia (p=0.012). When it comes to the frequency of thoracic and lumbar vertebral fractures, there was no difference between the groups as regards physical activity exercises and the daily calcium consumption (Table 2).
Clinical and demographic characteristics for the COPD group relating to normal bone mass, osteopenia and osteoporosis.
| Normal (n=19) | Osteopenia (n=45) | Osteoporosis (n=27) | p | |
|---|---|---|---|---|
| Sex | 0.82 | |||
| Male, n (%) | 12 (60) | 28 (59.6) | 18 (60) | |
| Female, n (%) | 7 (40) | 17 (40.4) | 12 (40) | |
| Age (years) | 61.9±9.2* | 66.4±8.5 | 70.3±9.1 | 0.008 |
| BMI (kg/m2) | 29.1±5.2 | 27.6±4.9 | 25.7±5.19 | 0.09 |
| Vertebral fractures, n (%) | 1 (5.9) | 10 (23.3) | 5 (19.2) | 0.29 |
| Fall with fractures, n (%) | 5 (26.3) | 17 (37.8) | 11 (40.7) | 0.57 |
| Calcium<1000mg/day, n (%) | 4 (21.1) | 14 (31.1) | 8 (29.6) | 0.68 |
| Spirometry | ||||
| FEV1 (%) | 56.4±22.7* | 60.8±22.2# | 44.6±15.2 | 0.007 |
| FVC (%) | 80.6±18.8 | 84.6±19.2 | 74.2±14.8 | 0.06 |
| FEV1/FVC | 52.5±14.0* | 52.9±12# | 44.6±10.9 | 0.01 |
| GOLD COPD stage, n (%) | ||||
| I/II | 13 (26.5) | 29 (59.2) | 7 (14.3) | 0.002 |
| III/IV | 6 (14.3) | 16 (38.1) | 20 (47.6) | |
| Inhaled corticosteroids | 15 (78.9) | 34 (75.6) | 26 (96.3) | 0.07 |
| Physical activity | 8 (42.1) | 19 (42.2) | 10 (37) | 0.91 |
Upon assessing clinical and demographic characteristics of only the control group regarding bone mass, we observed that the group with osteoporosis had a lower BMI than the normal group (Table 3).
Clinical and demographic characteristics of the control group relating to normal bone mass, osteopenia and osteoporosis.
| Normal (n=35) | Osteopenia (n=32) | Osteoporosis (n=15) | p | |
|---|---|---|---|---|
| Sex | 0.56 | |||
| Male, n (%) | 22 (62.9) | 18 (56.2) | 7 (46.7) | |
| Female, n (%) | 13 (37.1) | 14 (43.8) | 8 (53.3) | |
| Age (years) | 61.9±8.5 | 65.7±9.3 | 65.9±7.7 | 0.15 |
| BMI (kg/m2) | 30.7±3.7# | 27.9±4.0 | 29.2±5.3 | 0.02 |
| Vertebral fractures, n (%) | 2 (5.7) | 3 (9.7) | 2 (14.3) | 0.65 |
| Fall with fractures, n (%) | 2 (5.7) | 4 (12.5) | 0 (0) | 0.27 |
| Calcium≤1000mg/day, n (%) | 19 (54.3) | 16 (50) | 4 (26.7) | 0.19 |
| Spirometry | ||||
| FEV1 (%) | 99.7±11.4 | 100.5±18.1 | 104.1±18.5 | 0.66 |
| FVC (%) | 95.8±11.8 | 99.9±19.1 | 100.1±18.3 | 0.48 |
| FEV1/FVC | 80.4±5.5 | 78.8±6.3 | 81.4±5.7 | 0.32 |
| Physical activity | 17 (48.6) | 20 (62.5) | 4 (26.7) | 0.07 |
In the logistic regression analysis for osteoporosis development, we observed that osteoporosis was associated to the female gender, lower BMI values and being older (Table 4). However, for the presence of fractures the logistic regression analysis showed that female gender, higher BMI value and being older was associated to its development but interestingly COPD was not associated to fracture presence.
Regression model explaining the presence of osteoporosis.
| Coefficient | Exp (B) | 95% confidence interval | p | ||
|---|---|---|---|---|---|
| COPD | −0.42 | 1.55 | 0.37 | 6.34 | 0.57 |
| Male | −0.84 | 0.43 | 0.19 | 0.97 | 0.04 |
| Calcium≤1000mg | −0.227 | 0.797 | 0.37 | 1.69 | 0.555 |
| Age | 0.067 | 1.071 | 1.02 | 1.11 | 0.003 |
| BMI | −0.126 | 0.864 | 0.81 | 0.95 | 0.002 |
| Use of corticosteroids | −0.25 | 1.130 | 0.18 | 3.39 | 0.73 |
| Smoking | 0.275 | 0.760 | 0.056 | 10.344 | 0.94 |
| Physical activity | 0.272 | 1.313 | 0.612 | 2.815 | 0.66 |
| Constant | 0.53 | 1.65 | 0.81 | ||
We evaluated the prevalence of osteoporosis and vertebral fractures in patients with COPD and compared the results to a control group paired for age and sex. We found a higher prevalence of osteoporosis and thoracic vertebral fracture in the COPD group than in the control group. Osteoporosis in COPD was more frequent in older, severe/very severe patients and with a lower BMI, however when adjusted for age, sex and BMI the only factor that influenced the development of osteoporosis was age In the control group, a low BMI was associated to a higher frequency of osteoporosis.
The frequency of osteoporosis found in the COPD group was of 29.7%, similar to the 35% result found in a systematic review11 and lower than that found recently in a study carried out in Brazil, 42%,20 however, their patients were more severe (FEV1 41%). When we assessed bone mass loss, which reflects the association of osteopenia and osteoporosis, this value increased to 79.1%, corroborating Soriano et al.,22 who found a bone mass loss of 75% in patients with COPD.
COPD and osteoporosis share some common risk factors, such as smoking, low weight, poor nutrition and physical inactivity.23 The etiology of bone mass loss in COPD is yet not fully known; most probably it is multifactorial including factors such as female gender, use of corticosteroids, hypogonadism, smoking, physical inactivity, vitamin D deficiency, and chronic inflammation.4,24 We observed in our study that the higher osteoporosis frequency in the COPD group was associated to a lower BMI, but still within the normal range of normality, being older and having a more severe disease. Osteoporosis in the control group was associated only to a lower BMI value. In the logistic regression analysis the factors that were associated to osteoporosis were female gender, having a lower BMI and being older. In both groups, we did not find an association of osteoporosis with inactivity, smoking or low calcium ingestion. The logistic regression analysis for the presence of fractures did not show an association with COPD but it was associated to female gender, higher BMI value and being older. As expected female gender was significantly associated to osteoporosis (Table 4) but was also associated to the presence of fracture what is not usually commented. It would be interesting that vertebral fractures in female patients would be studied in larger samples to really confirm our finding.
It has already been described that a low BMI is associated to the presence of osteoporosis,5,25 despite this, in our study only one patient with COPD had a BMI value below normal. On the contrary, higher BMI was a protective factor for osteoporosis, what has already been described in a cross-sectional study.26 On the contrary, higher BMI was a risk factor for vertebral fracture. A possible explanation for the high BMI and fractures is that the high BMI is an extra and important load over a already fragile vertebra.
Regarding the presence of any vertebral fracture, the COPD group presented a 2.3 higher frequency than the control group, a value higher than that found in literature in which the risk of having a vertebral fracture is 1.6 greater in the COPD group.23 The frequency of vertebral fractures in the COPD group was 18.6% versus 9.0% in the control group, but could reach up to 40%, depending on the disease severity.23 Other factors involved in vertebral fracture are being older, a low BMI and the use of corticosteroids.27,28 Jorgensen et al.,24 found similar frequency of vertebral fracture (24%) in patients with COPD with a severity similar to that of our patients. The etiology of a fracture that arises from osteoporosis in patients with COPD is highly complex and multifactorial. Systemic inflammation is probably a determinant factor in the reduction of bone mineral density, as it happens in other chronic diseases.29
When we separated the frequency of vertebral fracture per region, thoracic or lumbar, the incidence was greater in the thoracic region with 17.4% in patients with COPD and 7.7% in the control group (p=0.06). Regarding the lumbar region, no statistical difference was seen between the frequency of 7.0% in the COPD group, and 1.3% in the control group (p=0.07). Fractures are associated to a great negative impact in quality of life and an increase in mortality in the population at large and this impact is even greater in patients with COPD.30 Patients with COPD and vertebral compression fracture present a higher number of hospitalizations, longer hospital stay and higher mortality.31
As this is a cross-sectional study, it was not possible to define the causes of osteoporosis in patients with COPD. The evaluation of the daily calcium consumption and the practice of physical activity was self-reported. Despite these limitations, due to the fact that we observed that the frequency of osteoporosis and of thoracic fracture were greater in patients with COPD than in the control group, we believe that evaluation of bone mineral density and the presence of thoracic fracture should be routinely included in clinical practice of COPD patients.
With this study, we observed that patients with COPD present a higher frequency of osteoporosis than a control group. Osteoporosis in the COPD group was associated to a lower BMI, being older and a more severe disease. When we evaluated all individuals with osteoporosis including both groups lower BMI and being older continued to be risk factors, however female gender now was also a risk factor for osteoporosis as has been shown. Despite the fact that osteoporosis was more prevalent in COPD patients than in the control group, when all risk factors are evaluated in an regression analysis, COPD turns not to be a risk factor “per se”. An early diagnosis of osteoporosis would allow an early treatment of this disorder probably avoiding vertebral fractures. A longitudinal study would be necessary to prove the efficacy of early diagnosis on preventing vertebral diagnosis.
Conflict of interestThe authors declare no conflict of interests.