Cystic fibrosis (CF) is a genetic multisystemic disease that causes the absence or alteration of the CF transmembrane conductance regulator (CFTR) protein, which functions as a chloride and bicarbonate channel. This disruption primarily affects the respiratory and digestive systems, leading to the accumulation of thick mucus and subsequent obstruction in these organs. CF is associated with significant medical complications, including recurrent respiratory infections, pancreatic insufficiency, malnutrition, and impaired lung function.
Considering possible causes, infertility or subfertility usually affects between 20 and 35% of women with CF. These causes include irregularities in the menstrual cycle, alterations in ovulation, delay in menarche or amenorrhea as a result of low weight, as well as, directly, by the expression of CFTR in the epithelial cells of the cervix and uterus. This results in a thick and dehydrated cervical mucus that acts as a barrier method for sperm penetration.
Pregnancy itself poses physiological challenges to the respiratory system, and the combination of hormonal changes, increased oxygen demand, and altered diaphragmatic mechanics can further strain lung function in women with CF. The introduction of CFTR modulators has provided new hope and possibilities in managing their condition during pregnancy. One of the key benefits of CFTR modulators is their potential to preserve and improve lung function, nutritional quality of life, and reduce respiratory and sweat chloride exacerbations.1–3 However, it is important to acknowledge that the potential teratogenic effects of CFTR modulators in pregnancy are currently unknown, primarily due to the lack of large observational studies.4,5
On the health of women with CF and on the newborn, some studies have shown little impact on survival in CF mothers with mild to moderate lung involvement. Factors such as diabetes, bronchial infection by certain pathogens, low weight, and lower maximum exhaled volume during the first second of forced exhalation (FEV1) than 50% could influence poorer results, although this has not been demonstrated in all series.6–8
Our main objective was to assess the influence of pregnancy on the clinical situation of CF patients, according to different clinical variables. Therefore, we carried out a retrospective study of 12 CF patients that had 15 pregnancies between 2006 and 2022, and gave birth to 16 babies (one dizygotic pregnancy).
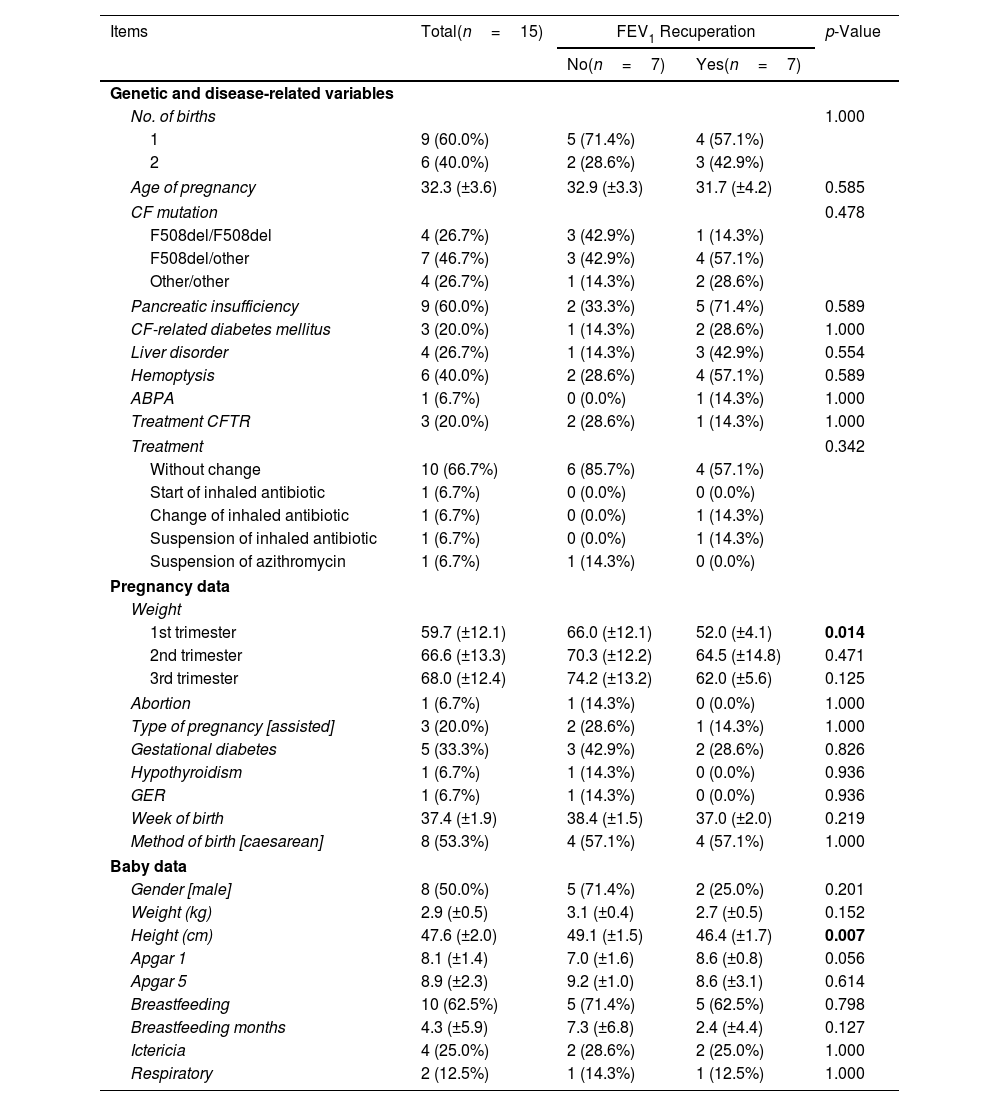
We conducted an initial descriptive analysis of the patients’ baseline values, their characteristics during pregnancy, and the characteristics of the newborns. The data was analyzed for the entire group of patients as well as for subgroups based on whether they achieved at least the baseline FEV1 after pregnancy or not (Table 1), from now on “recovered” and “non-recovered” groups. It is important to note that one patient could not be included in any of the groups due to the unavailability of her pre-pregnancy spirometry values, as per study restrictions.
Demographic and Clinical Characteristics of CF Patients in Total, and the Recovered and Non-recovered Groups.
| Items | Total(n=15) | FEV1 Recuperation | p-Value | |
|---|---|---|---|---|
| No(n=7) | Yes(n=7) | |||
| Genetic and disease-related variables | ||||
| No. of births | 1.000 | |||
| 1 | 9 (60.0%) | 5 (71.4%) | 4 (57.1%) | |
| 2 | 6 (40.0%) | 2 (28.6%) | 3 (42.9%) | |
| Age of pregnancy | 32.3 (±3.6) | 32.9 (±3.3) | 31.7 (±4.2) | 0.585 |
| CF mutation | 0.478 | |||
| F508del/F508del | 4 (26.7%) | 3 (42.9%) | 1 (14.3%) | |
| F508del/other | 7 (46.7%) | 3 (42.9%) | 4 (57.1%) | |
| Other/other | 4 (26.7%) | 1 (14.3%) | 2 (28.6%) | |
| Pancreatic insufficiency | 9 (60.0%) | 2 (33.3%) | 5 (71.4%) | 0.589 |
| CF-related diabetes mellitus | 3 (20.0%) | 1 (14.3%) | 2 (28.6%) | 1.000 |
| Liver disorder | 4 (26.7%) | 1 (14.3%) | 3 (42.9%) | 0.554 |
| Hemoptysis | 6 (40.0%) | 2 (28.6%) | 4 (57.1%) | 0.589 |
| ABPA | 1 (6.7%) | 0 (0.0%) | 1 (14.3%) | 1.000 |
| Treatment CFTR | 3 (20.0%) | 2 (28.6%) | 1 (14.3%) | 1.000 |
| Treatment | 0.342 | |||
| Without change | 10 (66.7%) | 6 (85.7%) | 4 (57.1%) | |
| Start of inhaled antibiotic | 1 (6.7%) | 0 (0.0%) | 0 (0.0%) | |
| Change of inhaled antibiotic | 1 (6.7%) | 0 (0.0%) | 1 (14.3%) | |
| Suspension of inhaled antibiotic | 1 (6.7%) | 0 (0.0%) | 1 (14.3%) | |
| Suspension of azithromycin | 1 (6.7%) | 1 (14.3%) | 0 (0.0%) | |
| Pregnancy data | ||||
| Weight | ||||
| 1st trimester | 59.7 (±12.1) | 66.0 (±12.1) | 52.0 (±4.1) | 0.014 |
| 2nd trimester | 66.6 (±13.3) | 70.3 (±12.2) | 64.5 (±14.8) | 0.471 |
| 3rd trimester | 68.0 (±12.4) | 74.2 (±13.2) | 62.0 (±5.6) | 0.125 |
| Abortion | 1 (6.7%) | 1 (14.3%) | 0 (0.0%) | 1.000 |
| Type of pregnancy [assisted] | 3 (20.0%) | 2 (28.6%) | 1 (14.3%) | 1.000 |
| Gestational diabetes | 5 (33.3%) | 3 (42.9%) | 2 (28.6%) | 0.826 |
| Hypothyroidism | 1 (6.7%) | 1 (14.3%) | 0 (0.0%) | 0.936 |
| GER | 1 (6.7%) | 1 (14.3%) | 0 (0.0%) | 0.936 |
| Week of birth | 37.4 (±1.9) | 38.4 (±1.5) | 37.0 (±2.0) | 0.219 |
| Method of birth [caesarean] | 8 (53.3%) | 4 (57.1%) | 4 (57.1%) | 1.000 |
| Baby data | ||||
| Gender [male] | 8 (50.0%) | 5 (71.4%) | 2 (25.0%) | 0.201 |
| Weight (kg) | 2.9 (±0.5) | 3.1 (±0.4) | 2.7 (±0.5) | 0.152 |
| Height (cm) | 47.6 (±2.0) | 49.1 (±1.5) | 46.4 (±1.7) | 0.007 |
| Apgar 1 | 8.1 (±1.4) | 7.0 (±1.6) | 8.6 (±0.8) | 0.056 |
| Apgar 5 | 8.9 (±2.3) | 9.2 (±1.0) | 8.6 (±3.1) | 0.614 |
| Breastfeeding | 10 (62.5%) | 5 (71.4%) | 5 (62.5%) | 0.798 |
| Breastfeeding months | 4.3 (±5.9) | 7.3 (±6.8) | 2.4 (±4.4) | 0.127 |
| Ictericia | 4 (25.0%) | 2 (28.6%) | 2 (25.0%) | 1.000 |
| Respiratory | 2 (12.5%) | 1 (14.3%) | 1 (12.5%) | 1.000 |
Data are shown as n (%), mean±standard deviation (SD). Significant comparisons (p<0.05) are marked in bold. 1st trimester: from week 0 to 12; 2nd trimester: from week 13 to 24; 3rd trimester: from week 25 to birth. CF: cystic fibrosis; ABPA: allergic bronchopulmonary aspergillosis; CFTR: cystic fibrosis transmembrane conductance regulator; BMI: body mass index; GD: gestational diabetes; GER: gastroesophageal reflux.
The group of patients who did not achieve FEV1 recuperation after pregnancy showed certain characteristics compared to the group that did achieve recuperation, although these differences were not statistically significant (p>0.05). The non-recovered group had a higher proportion of patients with only one child (71.4%) and a higher average age at pregnancy (32.9±3.3 years). Additionally, they had a lower proportion of heterozygous genotypes (57.1%) and lower incidences of pancreatic insufficiency (33.3%), cystic fibrosis-related diabetes mellitus (14.3%), liver disorder (14.3%), haemoptysis (28.6%), ABPA (0.0%), and CFTR treatment (28.6%).
Regarding treatments, there was no significant difference in the type of treatment between the two groups (p=0.342). The non-recovered group either maintained the same treatment or had a higher proportion of cases where azithromycin was suspended compared to the recovered group (85.7% vs 57.1% and 14.3% vs 0.0%, respectively).
In terms of weight and body mass index (BMI), there was an increase from the 1st trimester to the 3rd trimester (59.7±12.1 to 68.0±12.4, and 23.6±4.7 to 26.7±4.8, respectively), with the non-recovered group showing higher values. The difference in weight between the groups was statistically significant only in the 1st trimester (p=0.014).
Among the socio-demographic variables, there were no significant differences were observed between the groups (p>0.05). Regarding the baby data, most of the variables measured did not show significant differences between the groups (p>0.05), except for height, which was higher in the recovered group (49.1±1.5cm vs 46.4±1.7cm). However, variables such as the proportion of males, weight (kg), Apgar 5 scores, breastfeeding rates, breastfeeding duration, and the proportion of ictericia were higher in the group that did not achieve FEV1 recuperation, except for Apgar 1 scores, which were higher in the recovered group (7.0±1.6 vs 8.6±0.8).
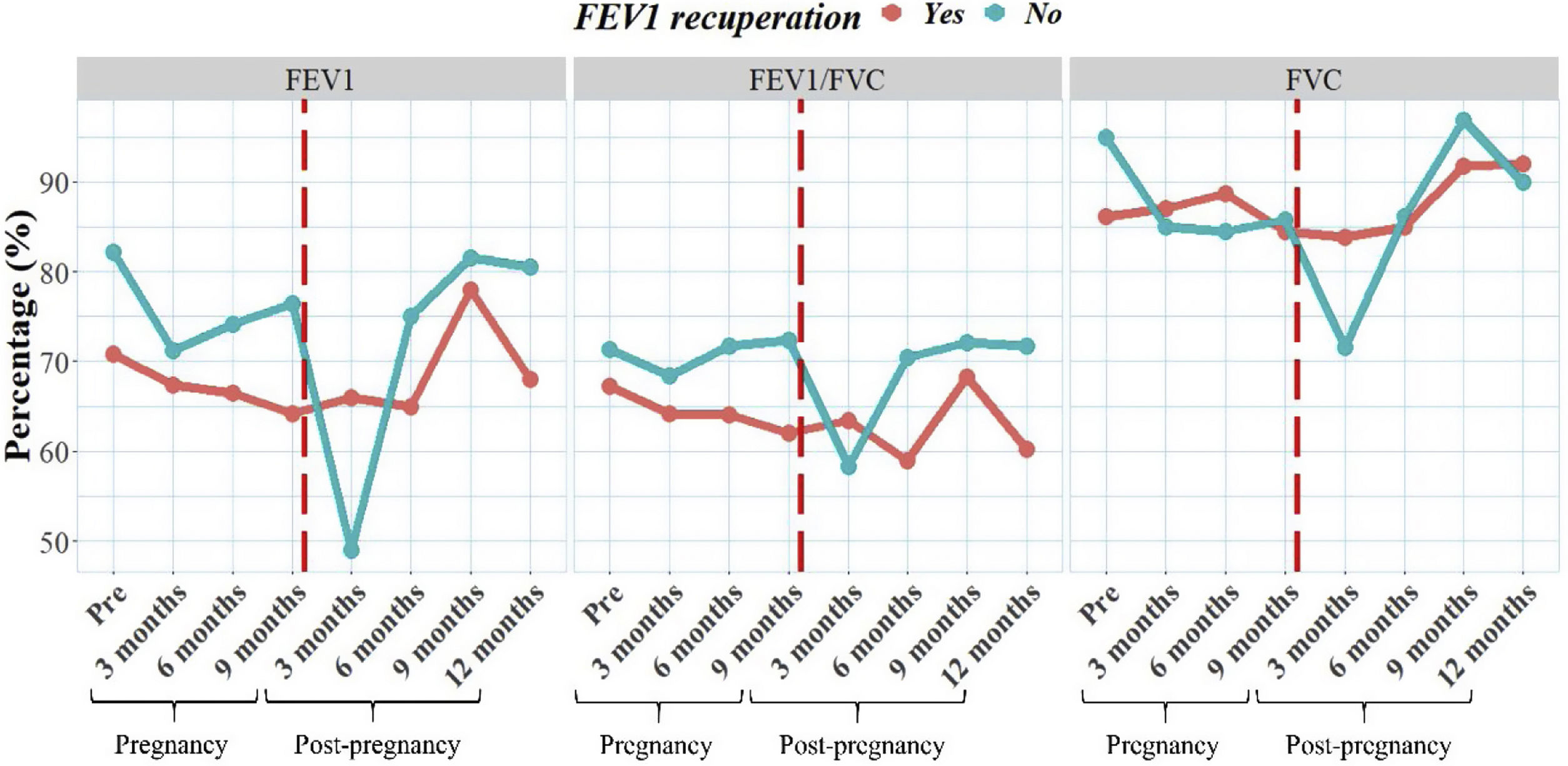
We also examined the progression of lung function for both groups over time, including data collected before pregnancy and at various intervals during and after pregnancy (Fig. 1). Our findings revealed differences in lung function between the groups; however, these differences were not statistically significant (p>0.05). Interestingly, the non-recovered group had higher FEV1 values before pregnancy compared to the other group. When analyzed the trend of spirometry values, we observed that the group with recovered group had more stable values over time, despite initially having lower values. In contrast, the non-recovered group experienced a significant decline in lung function at 3 months of pregnancy, which can be attributed to the physiological challenges faced during this trimester. Remarkably, this decline in lung function was not observed in the group that achieved recuperation.
This study showed a number of advantages and limitations in comparison with other studies. The lack of statistical significance may be attributed to the limited number of participants, making it difficult to detect meaningful differences between the groups. Another limitation is the non-standardized data collection process, which may have introduced variability in the measurements and affected the reliability of the results. The study would benefit from a larger sample size and a more rigorous data collection protocol to enhance the validity of the findings.
Despite these limitations, this study contributes to the limited body of research on the evolution of CF patients during pregnancy, shedding light on an understudied population. Despite the inherent limitations, the findings offer valuable insights into the unique challenges faced by CF patients during pregnancy. The need for further research with a larger sample size and standardized data collection is emphasized to obtain more robust and conclusive results. Future studies should adopt a prospective design, encompassing multiple hospitals, to increase the sample size and statistical power. Such endeavours would significantly advance our understanding of the impact of pregnancy on CF patients, leading to more precise recommendations for their management and care.
Conflict of InterestsThe authors state that they have no conflict of interests.