Cystic fibrosis causes exercise limitation due to impaired lung function and other complications, which in turn increases the chance of mortality. CFTR modulators, particularly the elexacaftor/tezacaftor/ivacaftor (ETI) combination, improve lung function in children older than 6 years in real-life studies.
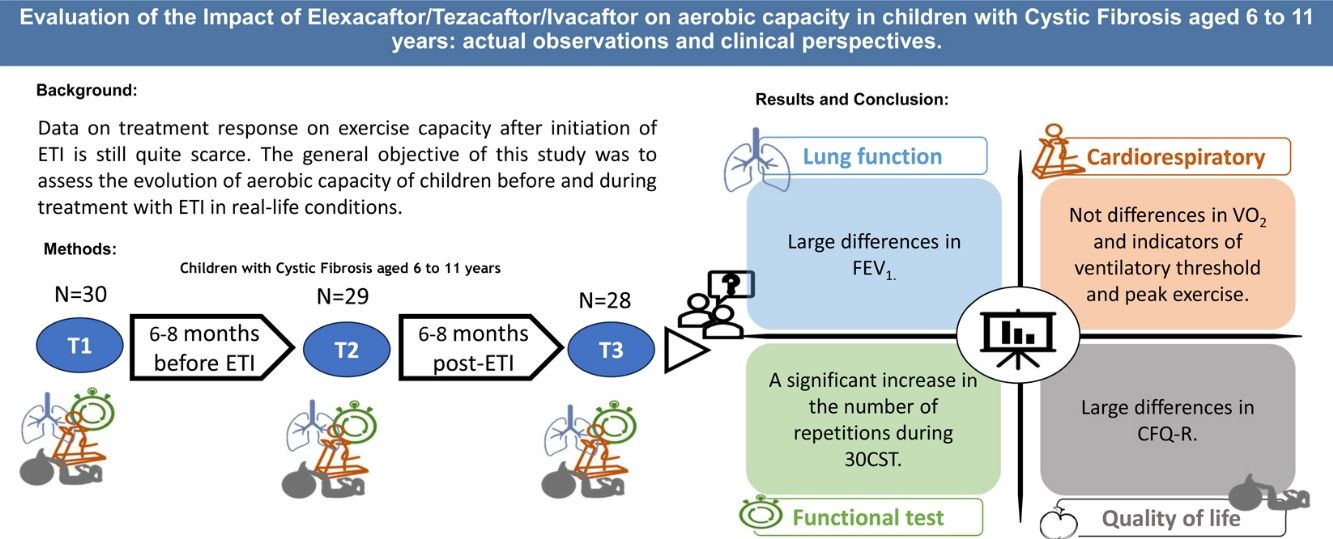
ObjectiveThis study aimed to assess the impact of ETI on aerobic capacity in children with CF aged 6–11 years under real-life conditions and to evaluate whether prior CFTR modulator treatment affects these outcomes.
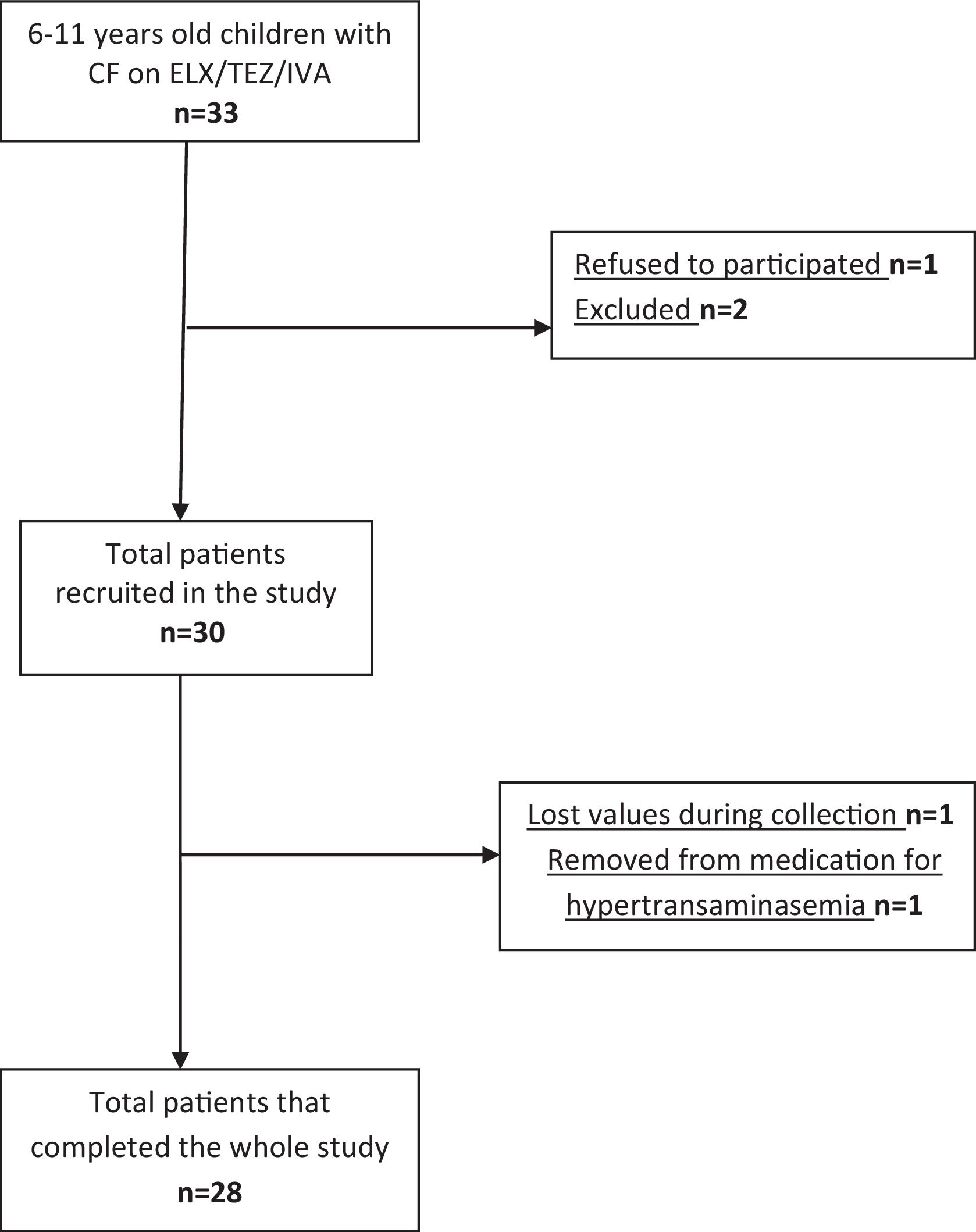
MethodsA multicenter, prospective cohort study was conducted with pediatric CF patients. Participants underwent evaluations 6–8 months before ETI (T1), at the start of ETI (T2), and 6–8 months post-treatment (T3). Primary outcomes included cardiorespiratory fitness assessed via peak oxygen consumption (VO2peak) during a cardiopulmonary exercise test (CPET), and secondary outcomes encompassed lung function, quality of life, physical activity, and functional mobility.
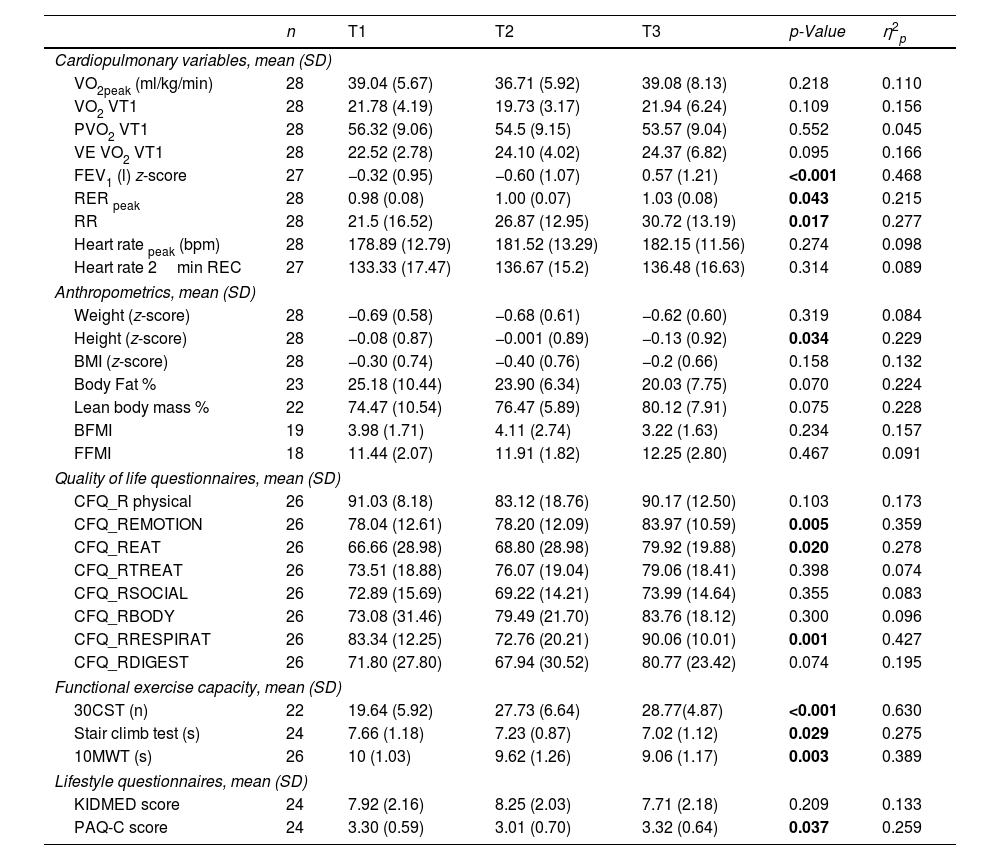
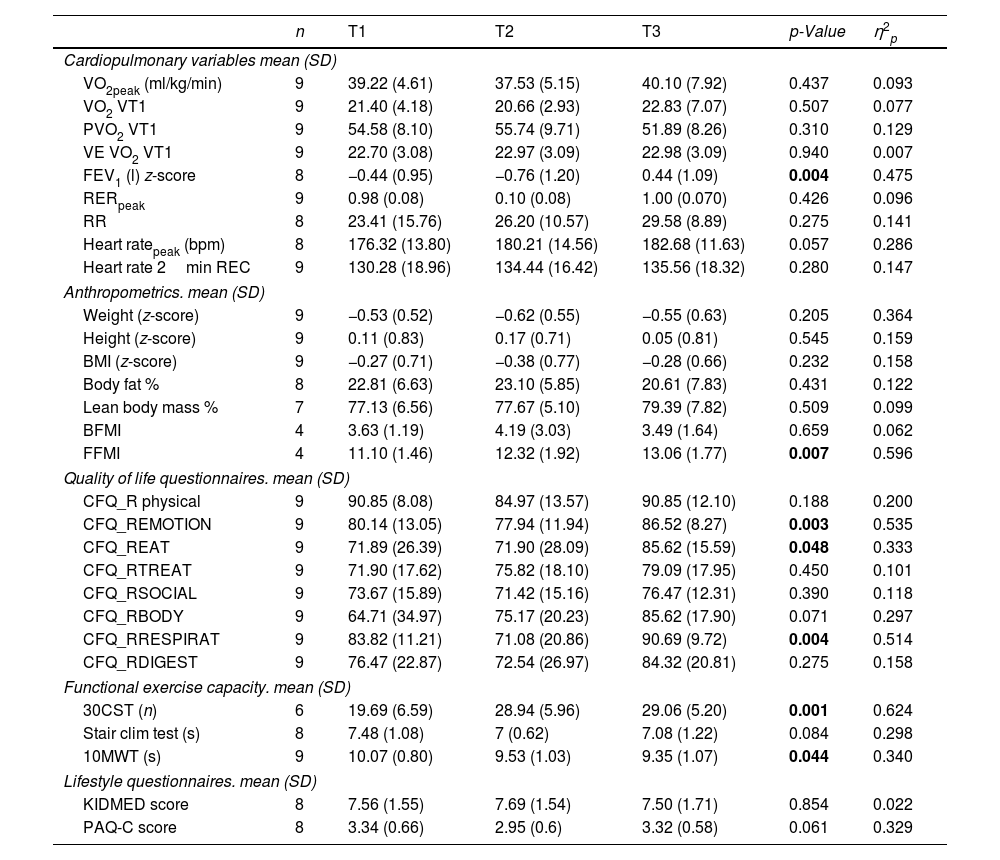
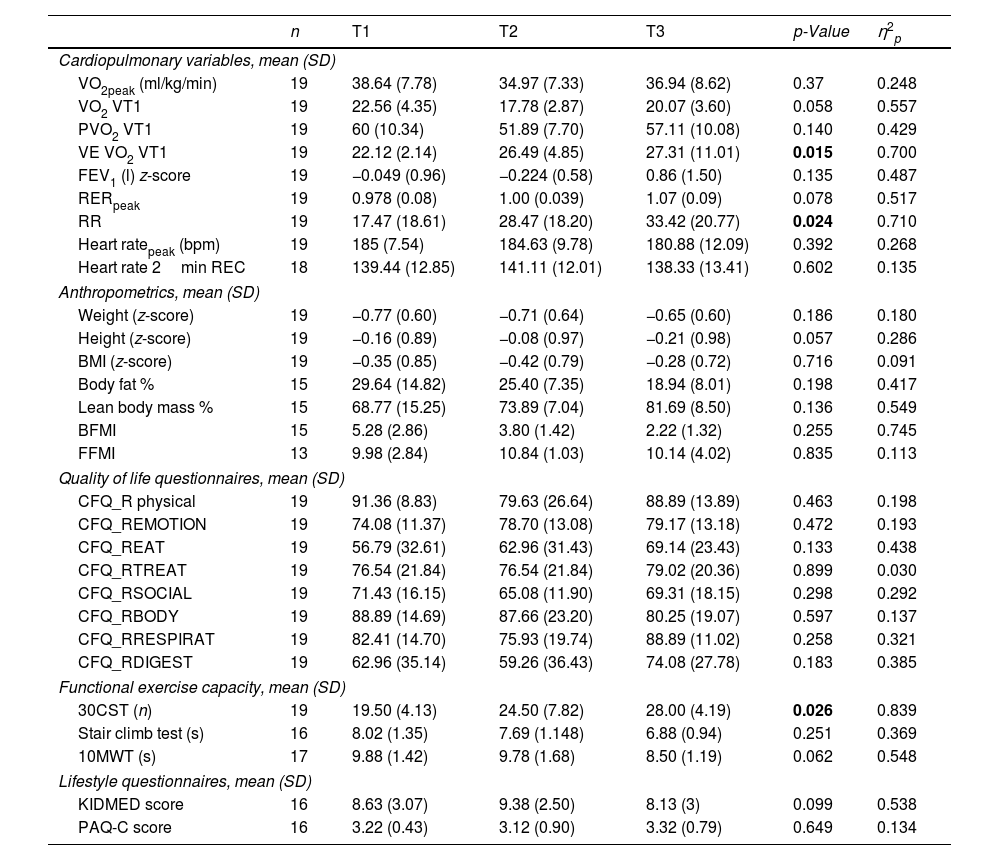
ResultsOf the 28 patients (mean age 9.02±1.59 years), 19 were ETI-naive, and 9 had prior CFTR modulator treatment. Significant improvements were observed in FEV1 (p<0.001), and several functional mobility tests (30CST, Stair Climb Test, 10MWT). However, VO2peak showed no significant changes between T1 and T3. Quality of life scores improved notably in emotional, eating, and respiratory domains, and a slight improvement was noted in physical activity levels (p=0.037).
ConclusionsETI treatment significantly enhances lung function and certain aspects of quality of life and physical fitness in pediatric CF patients. However, it does not significantly alter aerobic capacity (VO2peak) within the observed period.