The coexistence of bronchiectasis and asthma has been recognized for some time.1 In patients with severe asthma, it is estimated that between 30% and 50% may present with bronchiectasis as a comorbidity.2–5 This prevalence is notably higher among older individuals,2 those with more severe asthma,2,3 frequent sputum production,3 or concomitant gastroesophageal reflux disease.4 The presence of bronchiectasis in asthmatic patients has been associated with poorer lung function,5–7 increased exacerbation rates, and a higher frequency of hospitalizations.4–7
Biologic therapies have demonstrated efficacy in improving disease control in patients with severe, uncontrolled asthma.8 These therapies target key inflammatory pathways and have led to significant reductions in exacerbations, improvements in lung function, and better symptom control. However, it remains uncertain whether the presence of bronchiectasis alters the response to biologic treatment. While some studies suggest that bronchiectasis may be associated with a diminished therapeutic response,8 others report clinical improvement, including reduced exacerbation rates, in patients with both conditions receiving biologics.9
Given this uncertainty, the present meta-analysis aims to evaluate the efficacy of currently approved biologic therapies in patients with severe asthma and comorbid bronchiectasis, based on data derived from retrospective studies.
The systematic review protocol was registered in PROSPERO (CRD42024608643), and the study adhered to the PRISMA 2020 reporting guidelines10 (see checklist in the Supplementary Material).
We conducted a systematic literature search using Medline and Web of Science to identify studies evaluating the effect of biologic therapy in patients with severe asthma and comorbid bronchiectasis from the date of their inception to January 31, 2025, with no language restrictions. We used the search terms “asthma” AND “bronchiectasis” AND “biologic”. The search was independently performed by four reviewers. Discrepancies were resolved by consensus, and any unresolved disagreements were adjudicated by a fifth reviewer.
Inclusion criteria were: (a) studies on adult patients with severe uncontrolled asthma and coexisting bronchiectasis; (b) involving the use of any approved biologic therapy for asthma at the approved dose; (c) comparing symptoms, pulmonary function, and/or the number of exacerbations between treated and control groups, or during the year prior to and the year following the initiation of biologic therapy. There were no exclusion criteria.
The primary outcomes were changes in Asthma Control Test (ACT) scores, forced expiratory volume in the first second (FEV1), and the number of exacerbations over a one-year period.
For each included study, mean differences between baseline and 12-month follow-up were extracted for Asthma Control Test (ACT) scores, forced expiratory volume in one second (FEV1), and the annual number of exacerbations. When not directly reported, mean differences and standard deviations were estimated from the reported quantiles using the Box-Cox method or, in case of quantile ties or convergence errors, the Luo and Wan formula methods.11
A random-effects meta-analysis model was applied to account for potential inter-study heterogeneity. Pooled mean pre-post differences with corresponding 95% confidence intervals (CIs) were calculated for each outcome assuming a 0.5 correlation between baseline and 12-month follow-up. Statistical heterogeneity was assessed using the I2 statistic, with values >75% considered to indicate substantial heterogeneity.
All statistical analyses were performed using R (version 4.3) and the estmeansd and metafor packages. A two-tailed p-value <0.05 was considered statistically significant.
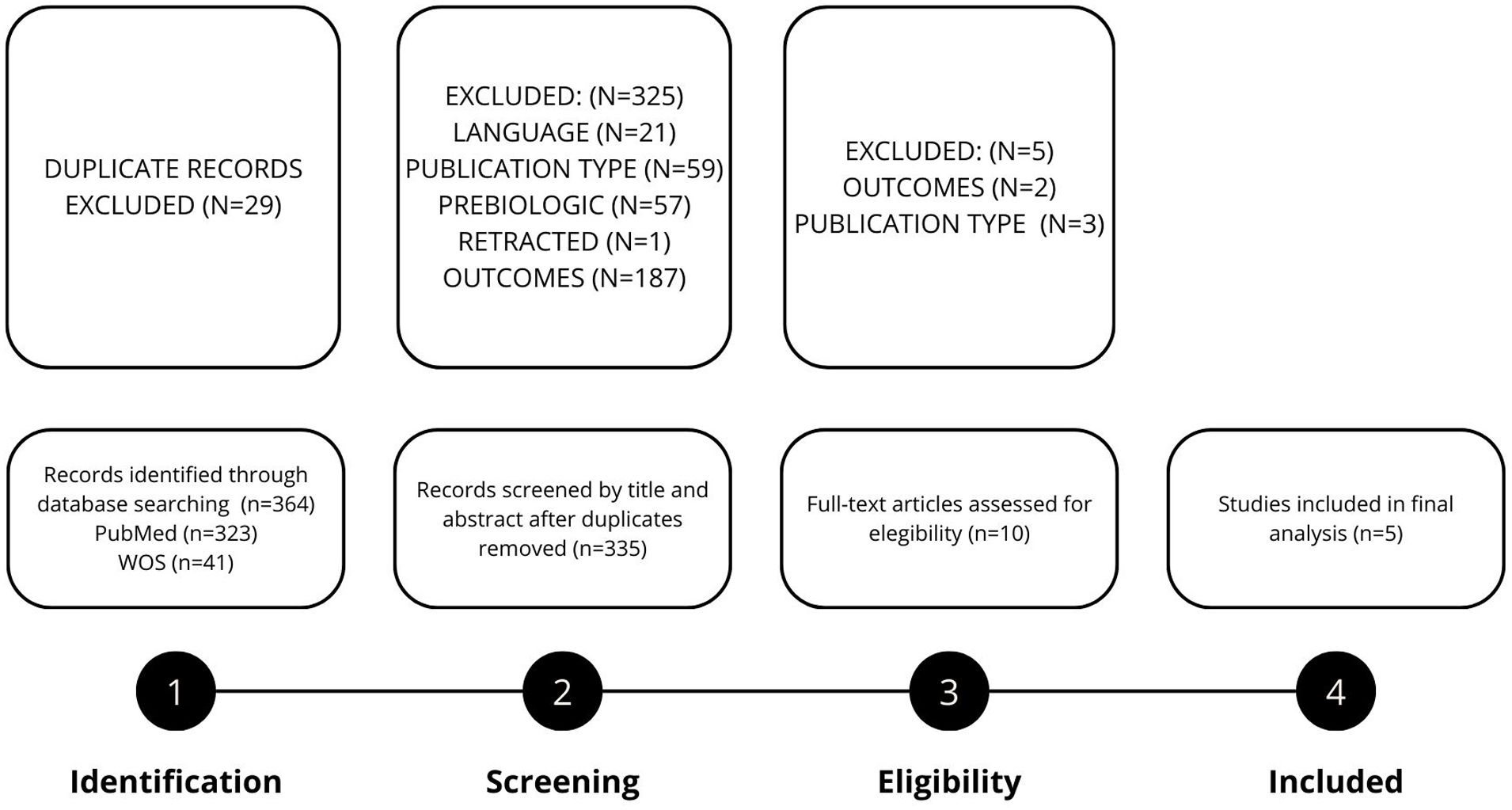
Fig. 1 presents the flow chart detailing the study selection process. A total of five studies were included in the final meta-analysis,12–16 encompassing 72 patients with severe asthma and comorbid bronchiectasis. All studies were retrospective, we did not find any randomized controlled trials (RCTs). The mean age of the study population was 56.1 years (SD 10.9), and 43 patients (59.7%) were female.
Regarding biologic therapy, 40 patients (55.5%) were treated with benralizumab, 23 (31.9%) with mepolizumab, 4 (5.5%) with dupilumab, and 2 (2.8%) with reslizumab. In addition, 3 patients (4.2%) received a combination of biologic therapies: 2 received benralizumab in combination with omalizumab, and 1 received dupilumab with omalizumab.
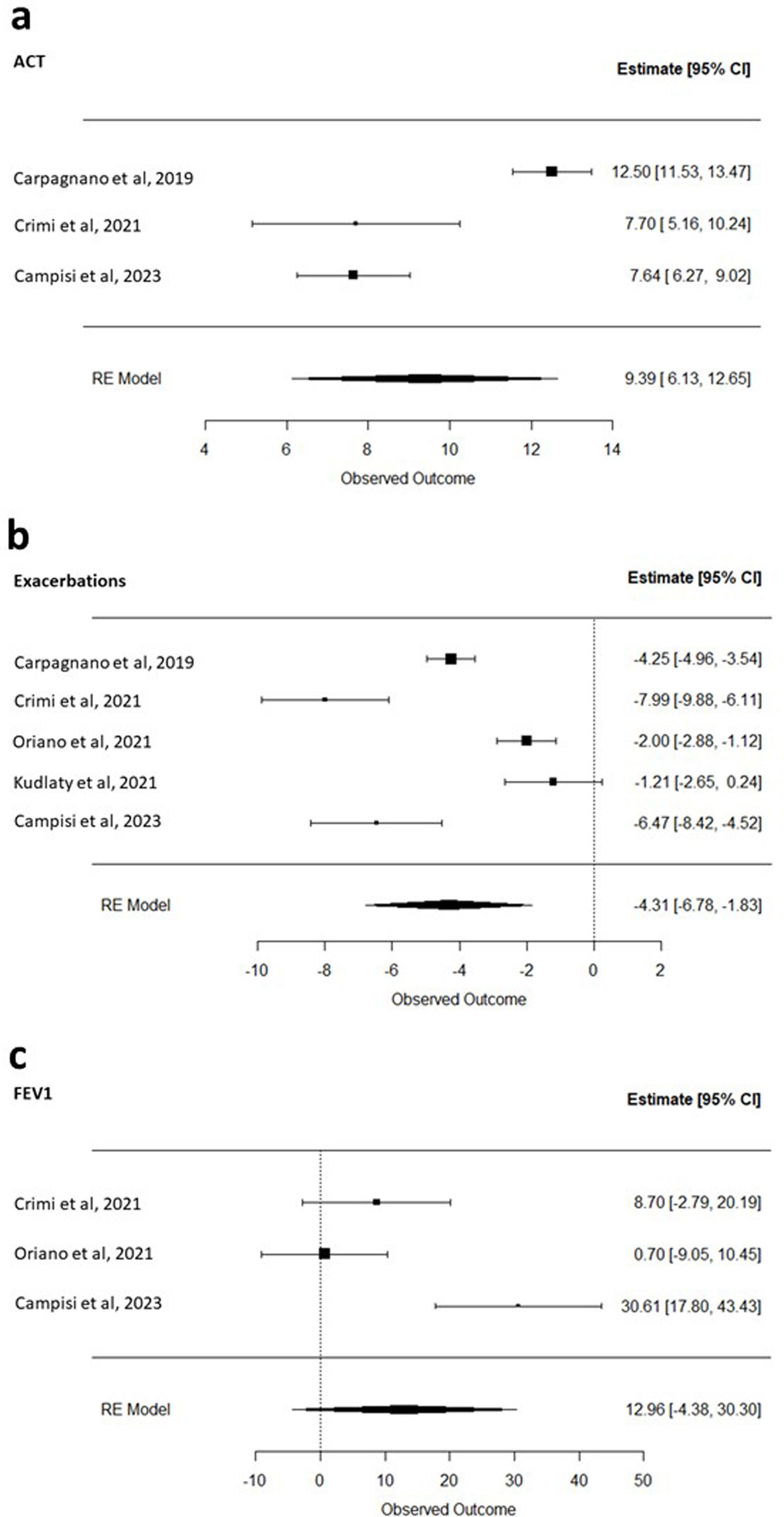
Three studies12,13,16 evaluated the impact of biologic therapy on Asthma Control Test (ACT) scores. Across these studies, treatment with biologics was associated with a significant improvement in ACT, with a pooled mean increase of 9.39 points (95% CI: 6.13 to 12.65; I2=93%) (Fig. 2a). All five studies included in the meta-analysis12–16 assessed the impact of biologic therapy on the number of exacerbations. Treatment with biologics was associated with a significant reduction in exacerbations, with a pooled mean decrease of 4.31 events per year (95% CI: −6.78 to −1.83; I2=95%) (Fig. 2b). Three studies13,14,16 evaluated the effect of biologic therapy on pulmonary function. In patients with asthma and bronchiectasis, biologic treatment was associated with an improvement in FEV1; however, the pooled mean increase of 12.96mL (95% CI: −4.38 to 30.30; I2=86%) did not reach statistical significance (Fig. 2c).
Forest plot of the (a) change in Asthma Control Test (ACT) scores before the initiation of biologic therapy and 12 months after treatment initiation; (b) reduction in asthma exacerbations before the initiation of biologic therapy and 12 months after treatment initiation; (c) increase in forced expiratory volume in one second (FEV1) before the initiation of biologic therapy and 12 months after treatment initiation.
This meta-analysis, which included five retrospective studies, demonstrated a reduction in the number of exacerbations and an improvement in symptom control, as measured by the ACT questionnaire, in patients with severe asthma and comorbid bronchiectasis after 12 months of biologic therapy. Although FEV1 showed a numerical increase, this improvement did not reach statistical significance.
The coexistence of bronchiectasis and asthma is not unexpected, as the two conditions may share common pathogenic mechanisms – whether genetic, environmental, or immunological. For instance, respiratory infections are considered key environmental triggers that may contribute to the development or worsening of either disease.1 As previously discussed, the presence of bronchiectasis in asthmatic patients is associated with poorer lung function, a higher rate of exacerbations, and increased hospital admissions.5,6 Furthermore, bronchiectasis has been linked to a greater frequency of pathogenic microorganism isolates in sputum, including Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Haemophilus parainfluenzae, and Aspergillus fumigatus.17
In our analysis, ACT scores improved significantly in the three studies that assessed this outcome.12,13,16 Additionally, all five studies included reported a decrease in the number of exacerbations following initiation of biologic therapy.12–16 These findings are consistent with those of a separate study reporting a reduction in exacerbation frequency after 12 months of treatment with mepolizumab, reslizumab, or benralizumab.9 However, this study could not be included in our meta-analysis because the number of exacerbations was reported as a categorical variable. Interestingly, the same study also documented a decrease in oral corticosteroid use following biologic therapy.
The study by Quaranta et al.18 was also excluded from our analysis, as it focused on different outcomes: the prevalence of clinical remission and predictors of response to biologic therapy in patients with asthma and bronchiectasis. Their results indicated that clinical remission occurred in 28.6% of patients and that baseline FEV1 was a significant predictor of remission.
On the other hand, several studies have suggested that the presence of bronchiectasis in asthmatic patients may not prevent response to biologics entirely, but may be associated with a relatively attenuated clinical effect.9,18 Rather than indicating treatment failure, bronchiectasis may act as a modifying factor, possibly due to more complex inflammatory profiles or underlying pathophysiological differences. Importantly, many of these studies did not consider the presence of chronic bronchial infection – a frequent comorbidity in bronchiectasis – that may influence treatment outcomes. For instance, Al-Ahmad et al. found that patients with bronchiectasis had a poorer response to omalizumab and benralizumab compared to those without bronchiectasis.8 Similarly, the “Southern Italy Network on Severe Asthma Therapy” has reported various clinical and biological factors associated with reduced response to biologics in this population.19–21 Di Bona et al. noted a higher prevalence of bronchiectasis among non-super-responders to benralizumab (28.9% vs 19.0%),19 that did not reach statistical significance, while Carpagnano et al. found that bronchiectasis was more frequent among patients who failed to achieve clinical remission (21.1% vs 13.6%),21 even though they did not find significant differences either. Finally, in a long-term study on clinical remission in severe eosinophilic asthma treated with mepolizumab, bronchiectasis – along with female sex, gastroesophageal reflux disease, and smoking history – was associated with a decrease in the achievement of long-term clinical remission.20 The fact that this lower response is not statistically significant in these studies – likely due to sample size – suggests that the associated worse prognosis may occur to a lesser extent than in other conditions, such as gastroesophageal reflux or reduced pulmonary function.
Our study has several limitations. All included studies were retrospective in design, inherently subject to selection bias and substantial heterogeneity. Furthermore, the lack of consistent data on respiratory pathogen isolates precluded a formal assessment of the role of chronic bronchial infection in modifying treatment response. Nevertheless, in the absence of randomized controlled trials, these retrospective studies represent the only available evidence and offer valuable insights into this understudied clinical scenario.
In conclusion, biologic therapies for severe asthma are associated with reduced exacerbation frequency and improved symptom control, as measured by the ACT questionnaire, in patients with coexisting bronchiectasis after one year of treatment. Well-designed randomized controlled trials are needed to better characterise the effectiveness of biologics in this subgroup of patients.
CRediT Authorship Contribution StatementStudy design: IGO, JR, AHB, NB
Data collecting: AHB, NB, DS, CMR
Statistical analysis: IGO, JR
Wrote the manuscript: IGO, CM
Read critically the manuscript: IGO, JR, AHB, NB, DS, CM
Declaration of Generative AI and AI-assisted Technologies in the Writing ProcessNone of the material has been produced with the help of any artificial intelligence software or tool.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of InterestThe authors do not have any financial or personal relationships with people or organizations that could inappropriately influence their work in the present article.