The prevalence of α1-antitrypsin PI*ZZ genotypes in patients with COPD is only partially known. We aimed to estimate this prevalence worldwide.
MethodA systematic review of the literature was conducted for studies investigating the prevalence of COPD and the prevalence of severe alpha-1-antitrypsin deficiency (AATD) PI*ZZ genotype. Results are shown in tables and on a whole world interpolation map.
ResultsStudies from 48 countries with available data (21 from Europe, 9 from the Americas, 5 from Africa, 11 from Asia and 2 from Australasia) were selected. About 235,000 individuals with PI*ZZ genotypes were accounted: 50% in Europe, 37% in America, 9% in Asia, 3% in Australasia and 1% in Africa. The estimated crude prevalence of COPD in adults older than 40 years was 12.45% in Europe, 13.51% in America, 13.22% in Africa, 11.70% in Asia and 11.86% in Australasia. The highest PI*ZZ weighted average prevalence among COPD subjects (expressed as 1/x [95% confidence intervals]) were found in Northern Europe (395 [252–576]) followed by Western (797 [538–1165]), Southern (944 [600–1475]) and Central Europe (1096 [687–1738]). Outside Europe, high values were found in Australia–New Zealand (1007 [684–1509]), Saudi Arabia (1276 [563–2961]), United States (1298 [1094–1540]), Canada (1482 [1057–2083]) and Thailand (1807 [717–4692]). In the rest of the world, prevalence was significantly lower, especially in vast regions of Asia and Africa where the PI*Z gene is practically non-existent.
ConclusionsSevere AATD is associated with a significant number of cases of COPD, especially in Europe, USA, Canada, New Zealand and Australia.
Chronic obstructive pulmonary disease (COPD) is a preventable but potentially life-threatening lung disease that affects over 380 million adults worldwide, with a high economic cost. In latest years, COPD accounted for 5% of all global deaths, with more than 90% occurring in low- and middle-income countries.1 In Spain, it affects 11% of adults over 40 years of age.2 Tobacco smoking is considered the major risk factor for developing COPD in developed countries.1 Other important risk factors for COPD are prolonged exposure to environmental contaminants, indoor and outdoor air pollutants, and ageing.1 In a meta-analysis of genome-wide association studies, several genetic loci were associated with COPD,3 however the only genetic risk factor clearly documented so far is the deficiency of the serine protease inhibitor SERPINA1, also called alpha-1 antitrypsin (AAT), a circulating glycoprotein secreted by liver that protects the connective tissue of the lungs from the proteolytic effects of neutrophil elastase.4
Severe α1-antitrypsin deficiency (AATD) is a rare underdiagnosed autosomal codominant genetic condition due to mutations of the SERPINA1 gene, which predisposes to the development of several types of respiratory and hepatic diseases.4 In clinical practice, 96% of severe AATD individuals with clinical manifestations are homozygous for the SERPINA1 PI*Z mutation (Glu342Lys), expressing a proteinase inhibitor PI*ZZ genotype, which is characterized by low serum concentrations of AAT of 10–15% and a loss of 80% of its inhibitory capacity.4
Lung emphysema and chronic bronchitis are the most common clinical phenotypes of COPD associated with PI*ZZ deficiency.5 Environmental factors, particularly cigarette smoke, greatly increase the risk of COPD development in patients with PI*ZZ genotype,4 and while the onset of respiratory disease in smokers occurs around the fourth decade of life, in no-smokers the onset can be delayed to the fifth or sixth decades, and remarkably between a third and slightly more than half of them remain without developing AATD-associated diseases throughout their lives.5 This clinical expression variability suggests that in a number of cases AATD alone is not enough to induce COPD, and that, in addition to smoking and other environmental pollutants, genetic and epigenetic modifiers, not yet identified, likely influence this variability.4 Actually, the genetic penetrance of the PI*ZZ genotype (that is, the proportion of individuals carrying the PI*ZZ genotype that expresses a COPD phenotype) is not well established, although it is known to be incomplete, being able to reach a percentage of up to 60%.6,7
Since AATD is the only subtype of COPD whose progression can be slowed significantly by lifelong augmentation therapy with infusions of purified plasma-derived AAT, it would be desirable to know the number of PI*ZZ subjects at high risk of developing COPD in each country, for planning health policies and financial medical resources.8,9 Therefore, using a similar methodology to that used by the authors in a previous meta-analysis on the same topic in Europe,10 we update and extend now our estimates to the remaining countries in the world with available data.
MethodsWe conducted a systematic literature search in Medline, EMBASE (through Ovid) and Google Scholar until December 2022, on both “number and prevalence of PI*ZZ genotypes” and “COPD prevalence” worldwide, selecting studies published in peer-reviewed journals, without language restrictions, to build a database of countries with reliable data in both epidemiological aspects. The methodology used to select and discard the collected articles has been described in previous publications on genetic epidemiology of AATD10–15 and COPD epidemiology,10,16–19 respectively.
Briefly, the following criteria were used for the selection of reliable papers on PI*ZZ deficiency: (1) samples of healthy unrelated people, representative of the general population; (2) AAT geno/phenotyping characterized by isoelectrofocusing or polymerase chain reaction techniques, and (3) results precision in accordance with a coefficient of variation derived from the sample size and 95% confidence intervals.11 Studies conducted with samples from patients with AATD-related diseases (e.g., COPD or liver cirrhosis) as well as screening studies, in which phenotypes were determined only in samples with AAT concentrations below any given cut-off point, were discarded.
The criteria for inclusion of articles to estimate the prevalence of COPD were: (1) cross-sectional and longitudinal prevalence surveys; (2) subjects of both sexes selected by simple random or stratified sampling); (3) mean age of the sample equal to or greater than 40 years old; (4) COPD diagnosis based on validated spirometry criteria. The criteria for exclusion of articles were: (1) randomized control trials or intervention studies; (2) samples not selected by random or stratified sampling; (3) samples of subjects with multiple diseases such as pulmonary tuberculosis, obstructive sleep apnoea, malignant tumours, etc.; (4) mean age of the samples below 40 years; (5) COPD diagnosis not confirmed by spirometry.
This methodology allowed obtaining the best available data on PI*ZZ and COPD prevalence from 48 of the 193 sovereign countries in the world. These countries were: (1) Denmark, Estonia, Finland, Latvia, Lithuania, Norway and Sweden from Northern Europe; Belgium, France, Netherlands, Republic of Ireland and United Kingdom from Western Europe; Austria, Germany, Poland and Switzerland from Central Europe; Italy, Portugal and Spain from Southern Europe; and European Russia and Serbia from Eastern Europe. (2) Canada, United States and México from North America. (3) Argentina, Brazil, Chile, Colombia, Uruguay and Venezuela from South America. (4) Morocco and Tunisia from Northern Africa; Cape Verde and Nigeria from Western Africa, and South Africa from Southern Africa. (5) Asian Russia, Iran, Israel, Jordan and Saudi Arabia from Northern and Western Asia; China, South Korea, Japan, Philippines and Thailand from East and Southeast Asia; and India for South Asia. (6) Australia and New Zealand from Australasia. Due to the long list of evaluated papers, a link to Supplementary material that includes references of the analyzed articles is provided.
Then, the following data were consecutively entered into a Microsoft Excel database: (1) total population and population aged ≥40 years from each selected country, according to the CIA's World Factbook [https://www.cia.gov/the-world-factbook] and the population pyramid distribution of the countries’ population by age groups [https://datosmacro.expansion.com/demografia/estructura-poblacion]. (2) Total number of PI*ZZ subjects of each country. (3) Number of PI*ZZ individuals aged ≥40 years, assuming a normal allelic distribution of AAT alleles in that age group.28 (4) Number of the PI*ZZ individuals aged ≥40 years resulting after the application of an agreed conversion factor of genetic penetrance of 60%. (5) Number of individuals with COPD aged ≥40 years, applying the percentage of COPD prevalence calculated for every country. (6) PI*ZZ/COPD ratio, calculated by dividing the total number of PI*ZZ individuals at high risk (i.e., those aged ≥40 years after applying a genetic penetrance factor of 60%) by the number of COPD subjects aged ≥40 years. (7) Finally, with the data of the previous calculations an inverse distance weighted (IDW) interpolation global map of PI*ZZ/COPD prevalence was obtained.
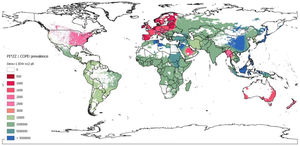
This IDW-interpolation map was created with the data provided by the countries with real data, while geographic areas without data were coloured using the Geographic Information Systems (GIS) technology. With this methodology virtually all regions of the world, including those without data, were dyed by interpolating the values of the neighbouring regions with real data. A continuous chromatic scale red-green-blue, in which red and blue expressed the maximumand minimum values, was chosen. The chromatic gradient scale corresponded roughly to the weighted average prevalence of PI*ZZ in the COPD population (1/x) of each country. Red colours represented the highest prevalence, i.e.: ≤1/500 (dark red) to 1/3000 (light red). Light greens corresponded to prevalences around 1/4000–10,000, and dark greens to prevalences of around 1:100,000. Light blues indicated very low prevalence (1/5,000,000), and dark blues corresponded to the lowest prevalence (>1/50,000,000, i.e.: 1/infinite or undefined prevalence). The areas with a population density of less than one inhabitant per square kilometre were hidden from visualization, appearing as white.
ResultsThe results are summarized numerically in Tables 1–6 and graphically in the GIS IDW-interpolation map of Fig. 1.
PI*ZZ and COPD Number, and PI*ZZ/COPD Prevalence in the Adult Population (Over 40 Years) From 21 European Countries.
| Region/Country | Population (n) | Population ≥40 Yrs (n) | Total PI*ZZ (n) | PI*ZZ ≥40 Yrs (n) | PI*ZZ ≥40 Yrs and GP 60% (n) | COPD Prevalence ≥40 yrs (%) | COPD ≥40 Yrs (n) | PI*ZZ/COPD Prevalence, ≥40 Yrs and GP 60%, 1:x [95% CI] |
|---|---|---|---|---|---|---|---|---|
| Northern Europe | ||||||||
| Denmark | 5,920,767 | 3,117,284 | 4090 | 2153 | 1292 | 18.2 | 567,346 | 439 [372–519] |
| Estonia | 1,211,524 | 710,323 | 752 | 379 | 227 | 11.5 | 81,687 | 359 [198–483] |
| Finland | 5,601,547 | 2,994,539 | 1929 | 1031 | 619 | 7.0 | 209,618 | 339 [273–421] |
| Lavtia | 1,842,226 | 1,041,703 | 4005 | 2265 | 1359 | 11.5 | 119,796 | 88 [41–196] |
| Lithuania | 2,683,546 | 1,552,140 | 717 | 415 | 249 | 11.5 | 178,496 | 717 [408–1270] |
| Norway | 5,553,840 | 2,437,490 | 1798 | 789 | 473 | 9.4 | 229,124 | 484 [356–659] |
| Sweden | 10,483,647 | 4,374,366 | 2262 | 944 | 566 | 11.5 | 503,052 | 888 [658–1200] |
| Subtotal/mean | 33,297,097 | 16,227,845 | 15,553 | 7976 | 4786 | 11.6 | 1,889,119 | 395 [252–576] |
| Western Europe | ||||||||
| Belgium | 11,180,840 | 4,123,468 | 3193 | 1178 | 707 | 9.3 | 383,483 | 543 [299–993] |
| France | 62,814,233 | 35,609,186 | 17,191 | 9746 | 5847 | 8.7 | 3,097,999 | 530 [409–687] |
| Netherlands | 17,400,824 | 8,047,389 | 5353 | 2476 | 1485 | 11.7 | 941,545 | 634 [365–1110] |
| Republic of Ireland | 5,275,004 | 2,512,484 | 2265 | 1079 | 647 | 8.0 | 200,999 | 311 [175–556] |
| United Kingdom | 67,791,400 | 30,266,462 | 13,044 | 5824 | 3494 | 16.8 | 5,084,766 | 1455 [930–2283] |
| Subtotal/mean | 164,462,301 | 80,558,989 | 41,046 | 20,301 | 12,181 | 12.05 | 9,708,791 | 797 [538–1165] |
| Southern Europe | ||||||||
| Italy | 61,095,551 | 36,051,020 | 10,652 | 6285 | 3771 | 15.1 | 5,443,704 | 1443 [985–2182] |
| Portugal | 10,242,081 | 5,423,476 | 4944 | 2618 | 1571 | 11.3 | 612,853 | 390 [193–803] |
| Spain | 47,163,418 | 25,970,362 | 13,065 | 7194 | 4317 | 11.8 | 3,064,503 | 710 [482–1048] |
| Subtotal/mean | 118,501,050 | 67,444,858 | 28,661 | 16,098 | 9,659 | 13.52 | 9,121,060 | 944 [600–1475] |
| Central Europe | ||||||||
| Austria | 8,913,088 | 4,905,136 | 1529 | 841 | 505 | 21 | 1,030,079 | 2040 [882–4829] |
| Germany | 84,316,622 | 47,464,663 | 20,611 | 11,603 | 6,962 | 14.7 | 6,977,305 | 1002 [653–1544] |
| Poland | 38,093,101 | 19,921,146 | 6791 | 3,551 | 2,131 | 13.2 | 2,629,591 | 1234 [802–1907] |
| Switzerland | 8,508,698 | 4,662,005 | 972 | 533 | 320 | 5 | 233,100 | 729 [331–1634] |
| Subtotal/mean | 139,831,509 | 76,952,950 | 29,903 | 16,528 | 9,917 | 14.13 | 10,870,076 | 1096 [687–1738] |
| Eastern Europe | ||||||||
| Serbia | 7,146,759 | 3,751,833 | 1159 | 608 | 365 | 9.7 | 363,928 | 998 [460–2201] |
| European Russia | 143,666,931 | 52,800,000 | 1653 | 607 | 364 | 9.7 | 5,121,600 | 14,063 [8257–24,044] |
| Subtotal/mean | 150,813,690 | 56,551,833 | 2812 | 1,215 | 729 | 13.60 | 5,485,528 | 7525 [3888–14,497] |
| Europe summary | 606,905,647 | 297,736,475 | 117,975 | 62,118 | 37,271 | 12.45 | 37,074,572 | 995 [639–1518] |
Abbreviations: n, number; ≥, symbol, equal or higher; PI*ZZ, protease inhibitor ZZ genotype; GP, genetic penetrance; COPD, chronic obstructive pulmonary disease; 95% CI: 95% confidence interval.
PI*ZZ and COPD Number, and PI*ZZ/COPD Prevalence in the Adult Population (Over 40 Years) From 9 Countries of America.
| Region/Country | Population (n) | Population ≥40 Yrs (n) | Total PI*ZZ (n) | PI*ZZ ≥40 Yrs (n) | PI*ZZ ≥40 Yrs and GP 60% (n) | COPD Prevalence ≥40 yrs (%) | COPD ≥40 Yrs (n) | PI*ZZ/COPD Prevalence, ≥40 Yrs and GP 60%, 1:x [95% CI] |
|---|---|---|---|---|---|---|---|---|
| North America | ||||||||
| Canada | 38,232,593 | 17,238,010 | 7181 | 3238 | 1943 | 16.7 | 2,878,748 | 1482 [1057–2083] |
| United States | 337,341,954 | 159,117,241 | 62,820 | 29,631 | 17,779 | 14.5 | 23,072,000 | 1298 [1094–1540] |
| Mexico | 129,150,971 | 44,867,056 | 3921 | 1362 | 817 | 7.8 | 3,499,630 | 4282 [2209–8386] |
| Subtotal/mean | 504,725,518 | 221,222,307 | 73,922 | 34,231 | 20,538 | 13.31 | 29,450,378 | 1434 [1160–1755] |
| South America | ||||||||
| Argentina | 46,245,668 | 17,555,608 | 1669 | 634 | 380 | 13.8 | 2,422,674 | 6373 [3289–12,484] |
| Brazil | 217,240,060 | 77,506,700 | 6162 | 2198 | 1319 | 15.5 | 12,013,539 | 9107 [4564–18,382] |
| Chile | 18,430,408 | 8,148,589 | 708 | 313 | 188 | 11.6 | 945,236 | 5033 [2620–9762] |
| Colombia | 49,059,221 | 19,158,503 | 1995 | 779 | 467 | 8.9 | 1,705,107 | 3648 [1916–7011] |
| Uruguay | 3,407,213 | 1,562,454 | 121 | 55 | 33 | 15.3 | 239,055 | 7180 [3682–14,243] |
| Venezuela | 29,789,730 | 9,303,887 | 1897 | 592 | 355 | 12.1 | 1,125,770 | 3167 [1764–5727] |
| Subtotal/mean | 364,172,300 | 133,235,741 | 12,552 | 4572 | 2743 | 13.85 | 18,451,381 | 6726 [3466–13,170] |
| America summary | 868,897,818 | 354,458,048 | 86,474 | 38,803 | 23,282 | 13.51 | 47,901,759 | 2057 [1560–2634] |
Abbreviations: n, number. ≥, symbol, equal or higher. PI*ZZ, protease inhibitor ZZ genotype; GP, genetic penetrance; COPD, chronic obstructive pulmonary disease; 95% CI: 95% confidence interval.
PI*ZZ and COPD Number and PI*ZZ/COPD Prevalence in the Adult Population (Over 40 Years) From 5 Countries of Africa.
| Region/Country | Population (n) | Population ≥40 Yrs (n) | Total PI*ZZ (n) | PI*ZZ ≥40 Yrs (n) | PI*ZZ ≥40 Yrs and GP 60% (n) | COPD prevalence ≥40 Yrs (%) | COPD ≥40 Yrs (n) | PI*ZZ/COPD Prevalence, ≥40 Yrs and GP 60%, 1:x [95% CI] |
|---|---|---|---|---|---|---|---|---|
| Northern Africa | ||||||||
| Morocoo | 36,738,229 | 12,697,228 | 44 | 15 | 9 | 12.6 | 1,599,851 | 175,342 [0–4227] |
| Tunisia | 11,896,972 | 4,571,398 | 0 | 0 | 0 | 7.4 | 338,283 | – |
| Subtotal/mean | 48,635,201 | 17,268,626 | 44 | 15 | 9 | 7.20 | 1,938,134 | – |
| Western Africa | ||||||||
| Cape Verde | 596,707 | 160,789 | 3 | 1 | 0 | 8.4 | 13,506 | 27,846 [597–] |
| Nigeria | 225,082,083 | 38,215,472 | 2340 | 397 | 238 | 9.3 | 3,554,039 | 14,909 [1988–145,366] |
| Subtotal/mean | 225,678,790 | 38,376,261 | 2343 | 398 | 239 | 9.30 | 3,567,545 | 14,936 [1970–145,918] |
| Southern Africa | ||||||||
| South Africa | 57,516,665 | 17,505,317 | 359 | 109 | 66 | 23.8 | 4,166,265 | 63,551 [0–66,323] |
| Africa summary | 331,830,656 | 73,150,204 | 2387 | 523 | 314 | 13.22 | 9,671,945 | – |
Abbreviations: n, number; ≥, symbol, equal or higher; PI*ZZ, protease inhibitor ZZ genotype; GP, genetic penetrance; COPD, chronic obstructive pulmonary disease; –, undefined; 95% CI: 95% confidence interval.
PI*ZZ and COPD Number, and PI*ZZ/COPD Prevalence in the Adult Population (Over 40 Years) From 11 Countries of Asia.
| Region/Country | Population (n) | Population ≥40 Yrs (n) | Total PI*ZZ (n) | PI*ZZ ≥40 yrs (n) | PI*ZZ ≥40 Yrs and GP 60% (n) | COPD Prevalence ≥40 Yrs (%) | COPD ≥40 Yrs (n) | PI*ZZ/COPD Prevalence, ≥40 Yrs and GP 60%, 1:x [95% CI] |
|---|---|---|---|---|---|---|---|---|
| Northern Asia | ||||||||
| Asian Russia | 39,390,205 | 20,482,907 | 96 | 50 | 30 | 21.8 | 4,465,274 | 149,081 [35,425–681,513] |
| Western Asia | ||||||||
| Iran | 86,758,304 | 25,007,049 | 3691 | 1064 | 638 | 18.7 | 4,676,318 | 7326 [2823–19,580] |
| Israel | 8,914,885 | 3,546,021 | 3 | 1 | 0 | 22 | 780,125 | – |
| Jordan | 10,998,531 | 2,608,657 | 0 | 0 | 0 | 8.2 | 213,910 | – |
| Saudi Arabia | 35,354,380 | 12,186,087 | 6555 | 2259 | 1356 | 14.2 | 1,730,424 | 1276 [563–2961] |
| Subtotal/mean | 142,026,100 | 43,347,814 | 10,249 | 3324 | 1995 | 17.07 | 7,400,777 | – |
| East Asia | ||||||||
| China | 1,410,539,758 | 680,944,940 | 0 | 0 | 0 | 13.6 | 92,608,512 | – |
| South Korea | 51,844,834 | 29,278,890 | 1932 | 1091 | 655 | 14.6 | 4,274,718 | 6530 [1251–39,424] |
| Japan | 124,214,766 | 77,691,827 | 4 | 3 | 2 | 8.6 | 6,681,497 | – |
| Subtotal/mean | 1,586,599,358 | 787,915,657 | 1936 | 1094 | 656 | 13.14 | 103,564,727 | – |
| Southeast Asia | ||||||||
| Philippines | 114,597,229 | 32,005,330 | 0 | 0 | 0 | 20.8 | 6,657,109 | – |
| Thailand | 69,648,117 | 35,476,857 | 8479 | 4319 | 2591 | 13.2 | 4,682,945 | 1807 [717–4692] |
| Subtotal/mean | 184,245,346 | 67,482,187 | 8479 | 4319 | 2591 | 16.80 | 11,340,054 | – |
| South Asia | ||||||||
| India | 1,389,637,446 | 448,210,056 | 495 | 160 | 96 | 7.4 | 33,167,544 | 346,240 [21,317–11,425,908] |
| Asia summary | 3,341,898,455 | 1,367,438,621 | 21,255 | 8947 | 5368 | 11.70 | 159,938,376 | – |
Abbreviations: n, number; ≥, symbol, equal or higher; PI*ZZ, protease inhibitor ZZ genotype; GP, genetic penetrance; COPD, chronic obstructive pulmonary disease; –, undefined; 95% CI, 95% confidence interval.
PI*ZZ and COPD Number, and PI*ZZ/COPD Prevalence in the Adult Population (Over 40 Years) From 2 Countries of Australasia.
| Region/Country | Population (n) | Population ≥40 Yrs (n) | Total PI*ZZ (n) | PI*ZZ ≥40 Yrs (n) | PI*ZZ ≥40 Yrs and GP 60% (n) | COPD Prevalence ≥40 Yrs (%) | COPD ≥40 Yrs (n) | PI*ZZ/COPD Prevalence, ≥40 Yrs and GP 60%, 1:x [95% CI] |
|---|---|---|---|---|---|---|---|---|
| Australia | 26,141,369 | 15,253,203 | 4126 | 2407 | 1444 | 12 | 1,830,384 | 1267 [918–1807] |
| New Zealand | 5,053,004 | 2,423,327 | 2216 | 1063 | 638 | 11 | 266,566 | 418 [248–709] |
| Australasia summary | 31,194,373 | 17,676,530 | 6342 | 3470 | 2082 | 11.86 | 2,096,950 | 1007 [684–1509] |
Abbreviations: n, number; ≥, symbol, equal or higher; PI*ZZ, protease inhibitor ZZ genotype; GP, genetic penetrance; COPD, chronic obstructive pulmonary disease; 95% CI: 95% confidence interval.
Summary of PI*ZZ and COPD Number and PI*ZZ/COPD Prevalence (1:x) in 48 Countries of the World.
| Region/Country | Population (n) | Population ≥40 Yrs (n) | Total PI*ZZ (n) | PI*ZZ ≥40 Yrs (n) | PI*ZZ ≥40 Yrs and GP 60% (n) | COPD Prevalence ≥40 Yrs (%) | COPD ≥40 Yrs (n) | PI*ZZ/COPD Prevalence, ≥40 Yrs and GP 60%, 1:x [95% CI] |
|---|---|---|---|---|---|---|---|---|
| Northern Europe | 33,297,097 | 16,227,845 | 15,553 | 7976 | 4786 | 11.64 | 1,889,119 | 395 [252–576] |
| Western Europe | 164,462,301 | 80,558,989 | 41,046 | 20,301 | 12,181 | 12.05 | 9,708,791 | 797 [538–1165] |
| Southern Europe | 118,501,050 | 67,444,858 | 28,661 | 16,098 | 9659 | 13.52 | 9,121,060 | 944 [600–1475] |
| Central Europe | 139,831,509 | 76,952,950 | 29,903 | 16,528 | 9917 | 14.13 | 10,870,076 | 1096 [687–1738] |
| Eastern Europe | 150,813,690 | 56,551,833 | 2812 | 1215 | 729 | 13.60 | 5,485,528 | 7525 [3888–4497] |
| Total Europe | 606,905,647 | 297,736,475 | 117,975 | 62,118 | 37,271 | 12.45 | 37,074,572 | 995 [639–1518] |
| North America | 504,725,518 | 221,222,307 | 73,922 | 34,231 | 20,538 | 13.31 | 29,450,378 | 1434 [1160–1755] |
| South America | 364,172,300 | 133,235,741 | 12,552 | 4572 | 2743 | 13.85 | 18,451,381 | 6726 [3466–13,170] |
| Total America | 868,897,818 | 354,458,048 | 86,474 | 38,803 | 23,282 | 13.51 | 47,901,759 | 2057 [1560–2634] |
| Northern Africa | 48,635,201 | 17,268,626 | 44 | 15 | 9 | 7.20 | 1,938,134 | – |
| Western Africa | 225,678,790 | 38,376,261 | 2343 | 398 | 239 | 9.30 | 3,567,545 | 14,936 [1970–145,918] |
| Southern Africa | 57,516,665 | 17,505,317 | 359 | 109 | 66 | 23.80 | 4,166,265 | 63,551 [0–66,323] |
| Total Africa | 331,830,656 | 73,150,204 | 2746 | 523 | 314 | 13.22 | 9,671,945 | – |
| Northern Asia | 39,390,205 | 20,482,907 | 96 | 50 | 30 | 21.80 | 4,465,274 | 149,081 [35,425–681,513] |
| Western Asia | 142,026,100 | 43,347,814 | 10,249 | 3324 | 1995 | 17.07 | 7,400,777 | – |
| East Asia | 1,586,599,358 | 787,915,657 | 1936 | 1094 | 656 | 13.14 | 103,564,727 | – |
| Southeast Asia | 184,245,346 | 67,482,187 | 8479 | 4319 | 2591 | 16.80 | 11,340,054 | – |
| South Asia | 1,389,637,446 | 448,210,056 | 495 | 160 | 96 | 7.40 | 33,167,544 | 346,240 [21,317–11,425,908] |
| Total Asia | 3,341,898,455 | 1,367,438,621 | 21,255 | 8947 | 5368 | 11.70 | 159,938,376 | – |
| Australasia | 31,194,373 | 17,676,530 | 6342 | 3470 | 2082 | 11.86 | 2,096,950 | 1007 [684–1509] |
| Total World | 5,180,726,949 | 2,110,459,878 | 234,792 | 113,860 | 68,316 | 12.16 | 256,683,602 | – |
Abbreviations: n, number; ≥, symbol equal or higher; PI*ZZ, protease inhibitor ZZ genotype; GP, genetic penetrance; COPD, chronic obstructive pulmonary disease; –, undefined; 95% CI, 95% confidence interval.
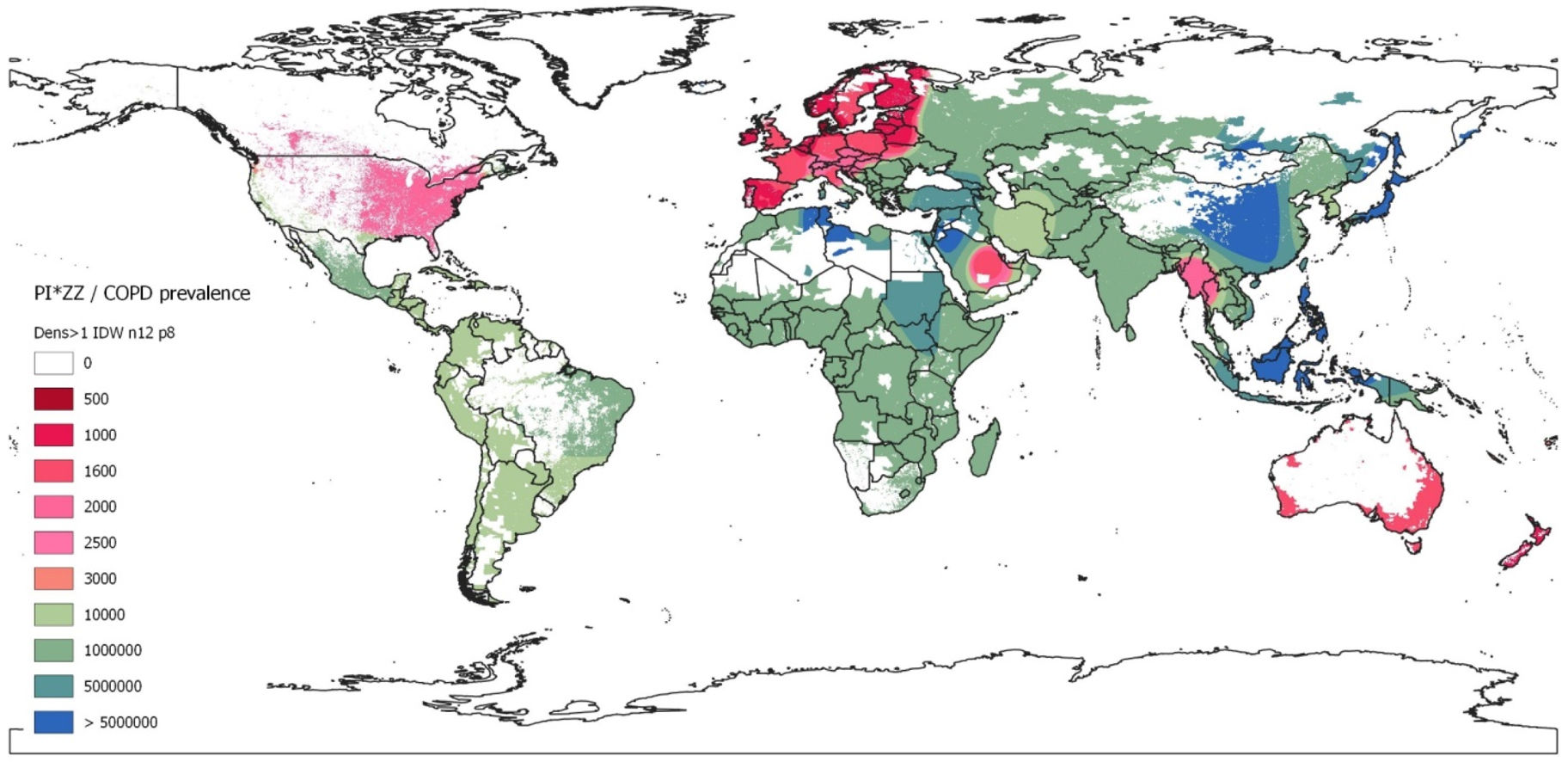
Map of inverse distance weighted interpolation (IDW) showing PI*ZZ/COPD prevalence (1/x) worldwide. The IDW interpolation map is based on the data obtained from 48 countries with available data. Geographic areas without real data were coloured using the Geographic Information Systems (GIS) technology. The colour scale corresponds to the weighted average prevalence of PI*ZZ in the COPD population (1:x) of each country. There is an approximate correspondence between the numerical values and the colours of the scale. Unpopulated or sparsely populated regions (with less than one inhabitant per square kilometre) are shaded white. Abbreviations: dens >1=population density greater than 1; n=number points with known values for IDW interpolation (12 for the current map); p=power parameter (p=8 for the current map).
In summary, we selected studies of 48 countries with available data (21 in Europe, 9 in the Americas, 5 in Africa, 11 in Asia and 2 in Australasia), with approximately (in round numbers) 5 billion people (62% of the approximately 8 billion of the world population). Of these 5 billion, approximately 2 (40%) were subjects over 40 years of age. Unfortunately, it was not possible to obtain reliable data from Central America and Caribbean islands nor from large regions of Africa and Asia (Table 6).
According to our calculations there are (approximately and in round numbers) 235,000 carriers of the PI*ZZ genotype among the 5 billion subjects of the selected countries (50% in Europe, 37% in America, 9% in Asia, 3% in Australasia and 1% in Africa), which would be reduced to 113,860 in the 2 billion population over 40 years of age, and these to 68,316 after applying a correction factor of genetic penetrance of 60%. The latter were distributed as follows: 37,271 (55%) in Europe; 23,282 (34%) in America (with 20,538 in North America, mostly Caucasian residents of the United States, and 2743 in South America); 5368 (8%) in Asia; 2082 (3%) in Australasia; and 314 (0.4%) in Africa (Table 6).
The crude prevalence of COPD in adults older than 40 years was 12.16%, distributed as follows: Europe 12.45%; North America 13.31%; South America 13.85%; Africa 13.22%; Asia 11.70%; and Australia–New Zealand 11.86%. These percentages presupposed the existence of approximately 257 million subjects with COPD distributed as follows: 160 in Asia; 37 in Europe; 29 in North America; 18 in South America; 10 in Africa; and 2 in Australia–New Zealand (Table 6).
The weighted mean prevalence of high-risk PI*ZZ genotypes among the adult population of subjects with COPD (1/x) could only be calculated for countries with values of PI*ZZ genotypes (Tables 1, 2 and 5), but it was incalculable for countries lacking PI*ZZ genotypes, because the 1/0 ratio is infinite or undefined (Tables 3 and 4).
By geographical regions, the highest PI*ZZ weighted average prevalence among COPD subjects (expressed as 1/x [95% confidence intervals]) was found in Northern Europe with one PI*ZZ every 395 COPDs (i.e., 395 [252–576]), followed by Western, Southern and Central Europe with 797 [538–1165]; 944 [600–1475]; and 1096 [687–1738], respectively. Outside Europe, high values were found in New Zealand (418 [248–709], Australia 1267 [918–1807], Saudi Arabia (1276 [563–2961]), United States (1298 [1094–1540]), Canada (1482 [1057–2083]) and Thailand (1807 [717–4692]). Lower prevalences ranging between 1/3000 and 1/9000 were found in Mexico, South America, Iran and South Korea. In the rest of the world, prevalence was significantly lower, especially in vast regions of Asia and Africa where the PI*Z gene is practically non-existent (Table 6).
For a quick overview these results are graphically represented on the coloured map of Fig. 1.
DiscussionOur results show that there is a remarkable uneven distribution of the prevalence of PI*ZZ genotypes among COPD subjects around the world. Summarizing, the highest prevalence of PI*ZZ genotypes in COPD patients was found in Europe, with the highest values in the Nordic regions, gradually decreasing towards the West, South and Centre, and sharply declining towards the East of the continent. Outside Europe, high and moderate prevalences were found in New Zealand, United States, Canada, Australia and Thailand. Lower but not irrelevant prevalences were found in South America, South Korea, Saudi Arabia and Iran. In all other countries prevalence values were much lower or even non-existent, especially in large parts of Africa and Asia. No numerical data were available for Central America or the Caribbean islands, which, however, were automatically dyed in the IDW-interpolation map with a light green colour, indicative of a moderate prevalence.
It has been reported that the Z mutation was generated 2000–3000 years ago in the southern Scandinavian Peninsula, from where it was spread to the rest of the European populations, and their descendants took it to the countries to which they emigrated or colonized, especially America, Australia and New Zealand.21 In general, our results are consistent with that theory, but not with the finding of some significant prevalence of PI*ZZs in Thailand, Saudi Arabia, South Korea or Iran, a fact consistent with the de Serres 2002 saying that “AAT deficiency is not just a disease of whites in Europe, but that it affects individuals in all racial subgroups worldwide”.22
In this context, we can mention that as well as the PiZ variant the infrequent “rare” and “null” variants have been diagnosed mainly in Caucasians of European heritage; however some other severe deficient variants or mutations have also been described in subjects of other races and ethnicities from many parts of the world. For example, the PI*Siiyama and PI*Mnichinan variants are practically exclusive to Japanese23,24; a severely deficient heterozygous Siiyama/QOClayton genotype has been described in Korea25; the PI*Mmineral springs genotype has been found only in a black family26; in Africans from Tunisia, the rare deficient alleles PiMmalton, PiPlowel and PiMwurzburg outnumber PiZ,27 and in a recent study from Turkey several rare deficient variants outnumbered the Z variant.28
The first documented complete deletion of the coding exons of the AAT gene was demonstrated by Takahashi and Crystal in Bethesda (Maryland, USA) in 1990 in the QOisola di Procida allele.29 Since then, molecular diagnosis of rare, dysfunctional and null variants has increased significantly, and currently 50 dysfunctional and deficient types of variants and 48 null variants have been reported all over the world.30 Although these are generally isolated cases or small series, rare and null deficient variants might be more frequent than expected anywhere in the world, coinciding with the increase in the index of suspicion for AATD and the more generalized use of advanced molecular diagnostic techniques.31
The presence of AATD must be investigated in all patients with COPD and blood relatives of index cases, as well as in any other pathology related to AATD.4,6 In a national screening carried out in Italy in 2005 of 2922 subjects, a total of 155 individuals with severe AATD were detected, most of them PI*SZ and PI*ZZ, but about thirty individuals carrying one rare or null variant in homozygosis or heterozygosis.32 In Ireland, in a national screening of 3000 individuals with clinical data suspicious for AATD, 816 deficient genotypes, including 42 ZZ and 23 rare mutations were detected.33 In 2010, an AATD screening programme targeting patients with respiratory disorders was initiated in Poland, achieving a detection of PiZZ homozygotes 16 times higher than that of the general population.34 In a national screening conducted in Germany between 2003 and 2015, 18,638 dried blood samples were analyzed and 6919 patients with at least one deficient allele were identified, including 271 individuals with various rare genotypes.34 In Spain, using buccal swabs and dried blood samples from 5803 patients, mostly with COPD, and relatives of AATD patients, the prevalence of SZ and ZZ was 3.7% and 1.4% respectively; in addition, 209 carriers of rare alleles, 12 carriers of null alleles and 14 new mutations were identified.35 In a multinational study on dried blood spots and buccal swabs of 30,827 samples from subjects with suspected AATD, conducted in Argentina, Brazil, Chile, Colombia, Spain, and Turkey, the prevalence of SS, SZ and ZZ was 0.9%, 1.9% and 0.9%; additionally, 70 new mutations were identified, with a surprising preponderance of the rare Plowell and Mmalton genotypes in Turkey.36,37 Finally, in the recently founded European AATD Research Collaboration (EARCO) international registry, currently with 1,044 registered individuals from 15 countries, the most frequent genotypes were PI*ZZ (60%), PI*SZ (29%), PI*SS (3.9%) and several rare variants (6.6%).7
Despite these fruitful initiatives they are still insufficient, and in many other countries most of those affected by AATD remain undiagnosed and consequently without specific care and treatment. Potential benefits of an early diagnosis include genetic counselling, lifestyle recommendations (such as smoking prevention or cessation, avoidance of high-risk occupations), augmentative therapy evaluation, and the possibility of taking advantage of future advances that can be provided by the various clinical trials currently under development.9
In the present study, despite the fact that the data of PI*ZZ and COPD prevalence rates were obtained with the best available evidence, the authors are aware that some might be biased due to the inherent limitations of this kind of epidemiological studies. Therefore, some of the provided results should be considered as indicative and handled with caution. For example, the selected studies on genetic epidemiology of the PiZ allele may not be fully representative because of the heterogeneous composition of the samples, mainly blood donors and new-borns, as well as a diverse group of individuals labelled under the generic title of “healthy unrelated people”, who included, school or college students, soldiers, hospital staff, routine medical examinations, active workers, and so on.10–15 Another confounding factor is the distribution of the studies and their number, most of them conducted in Europe and America, and only a few in Africa and Asia, as well as the different periods of time of their realization.10–15 In addition, possible biases attributable to the COPD studies include: unequal proportion of women and men in the samples studied; no uniformity of the subjects’ age; lack of proportionality between urban and rural samples; different exposure of the subjects to environmental, labour or domestic pollutants; and lack of uniformity in the technique interpretation and case definitions of spirometry measurements.10,18–20 For example, the definition based on post-bronchodilator FEV1/FVC<0.70 was used in the majority of the selected surveys (i.e. 82%), but this still does not address all possible sources of variation in case definition, since this fixed ratio criterion may potentially over-diagnose COPD in the elderly and it may under-diagnose COPD in younger patients.38 Furthermore, although GIS is a powerful tool to convey the epidemiology of respiratory diseases, given the scarcity and possible biases of some epidemiological data entered into the software database, it cannot be ruled out that some errors may have been generated in map shading.39
In summary, despite of the aforementioned limitations, our study provides an approximate overview of the prevalence of the PI*ZZ genotype in the COPD population worldwide. These data may be of interest to increase the interest of health care providers, the general public and patients to reduce under-diagnosis and encourage research about this rare disease.
Conflict of InterestsMarc Miravitlles has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Kamada, Takeda, Zambon, CSL Behring, Specialty Therapeutics, Janssen, Grifols and Novartis, consulting fees from AstraZeneca, Atriva Therapeutics, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, CSL Behring, Inhibrx, Ferrer, Menarini, Mereo Biopharma, Spin Therapeutics, ONO Pharma, Palobiofarma SL, Takeda, Novartis, Novo Nordisk, Sanofi, Zambon and Grifols and research grants from Grifols. The remaining authors report no conflicts of interest in this work.