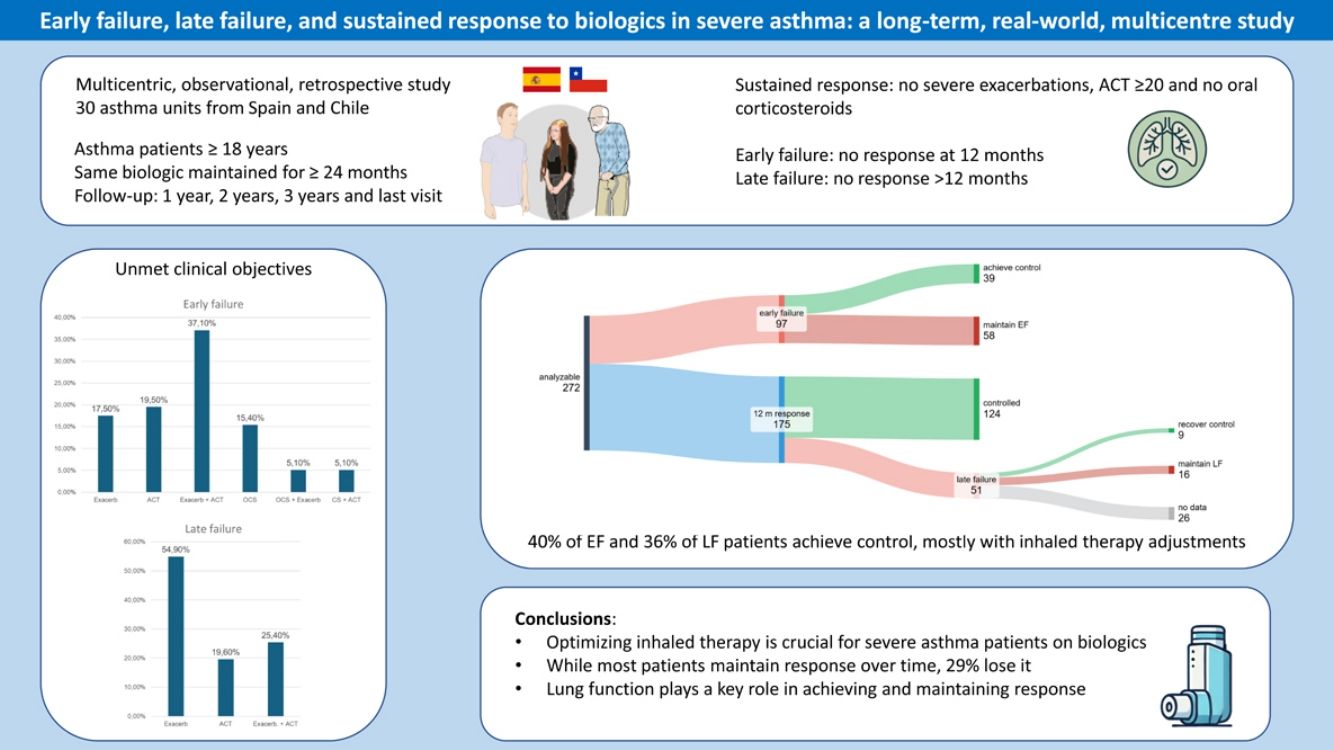
Only one-third of patients with severe asthma (SA) achieve a complete response to biologics. This study aims to characterize two types of failure: early (EF), occurring ≤12 months after biologic initiation, and late (LF), occurring at any time during follow-up after response has been achieved at 12 months.
MethodsThis is a multicentre retrospective study of adults treated with the same biologic for ≥24 months. Response was defined as no severe exacerbations in the preceding 12 months, asthma control test ≥20, and no need for maintenance oral corticosteroids. Failure (EF or LF) was defined as non-achievement of any of these objectives.
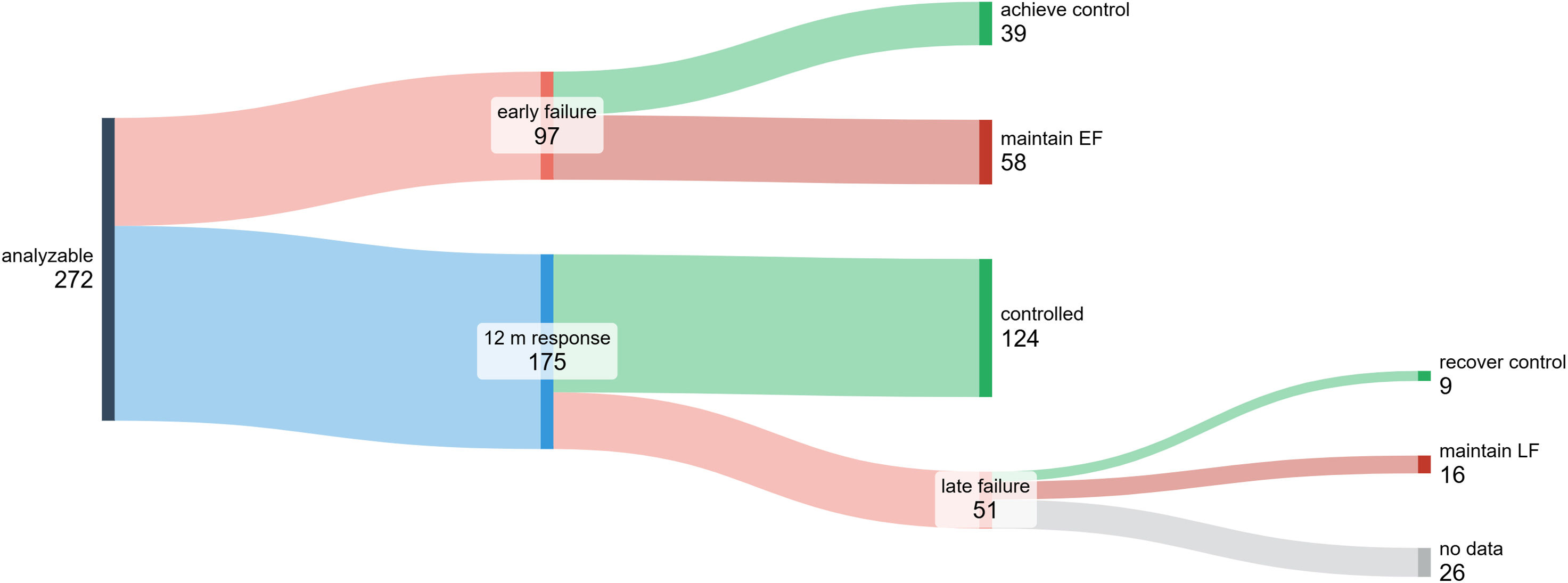
ResultsTwo hundred and seventy-two patients were analysed with a mean follow-up of 46.1±19.4 months. At 12 months, 97/272 were classified as PF, but 40% of them recovered response on subsequent visits (by changing inhaled therapy in 74%). Among the 175 responders at 12 months, 124 (70.8%) maintained response throughout the study period, while 51 (29.1%) experienced SF; those patients had lower FEV1 values after 12 months of biological therapy. SF reverted in 36% of cases, with inhaled therapy changes in 41.6%. FEV1 decreased by ≥100mL in 12 of 16 cases who did not recover response after SF.
ConclusionMost patients who achieve response at 12 months maintain it over time, but 29% of them suffer LF. Optimization of inhaled therapy can aid response recovery from EF or LF. Maximizing pulmonary function helps to prevent loss of response.
Severe asthma (SA) affects up to 3.6% of adults and 2.5% of children with asthma, with notable variability between countries.1,2 SA poses a significant disease burden, including potentially life-threatening exacerbations and reduced quality of life.3 The availability of biologics like omalizumab, mepolizumab, reslizumab, benralizumab, dupilumab, and tezepelumab has greatly improved prognosis.4 Nonetheless, real-life studies indicate only about one-third of patients achieve a complete response, however defined.4–10
In other fields, such as Rheumatology, treatment failure has been categorized into primary and secondary failure, with primary failure indicating lack of clinical response during the initial treatment period and secondary failure indicating loss of effectiveness over time.11,12 Primary failure may arise from targeting the wrong therapeutic pathway, while secondary failure is often driven by anti-drug antibodies.13 Anti-drug antibodies have been shown to negatively impact biologic response in rheumatoid arthritis, with responders having higher drug concentrations than non-responders.14
No distinction between these temporary types of failure has yet been made in SA patients on biologics, though both commonly arise in clinical practice. By convention, and in order to cover all seasons (seasonality may influence the occurrence of exacerbations), a 12-month period has been established to assess the response to a biologic. Therefore, it seems appropriate to classify failure as early (≤12 months) or late (>12 months). While early failure (EF), or nonresponse, is well-defined, criteria for late failure (LF) remain unclear, complicating decisions on dose adjustment or switching biologics. Identifying risk factors and distinguishing characteristics between patients with LF and sustained response may help predict LF.
This study aims to assess whether significant differences exist in the characteristics of patients experiencing sustained response, EF, and LF, whether EF and LF are permanent or may reverse over time, and whether patients presenting with these two types of failure exhibit differential clinical characteristics.
Material and MethodsDesignThis observational, retrospective, multicentre study was conducted across 29 Spanish Asthma Units and one in Chile. An electronic data collection repository was created to include data from patients who attend or attended the asthma clinics of the participating centres and who met the inclusion-exclusion criteria of this study. Investigators collected data from patients’ electronic medical records, using the date of biologic prescription as the index date. Data were gathered closest to 12, 24, and 36 months post-index date, and at the last asthma clinic visit (if the date exceeded 36 months post-index). The study was approved by the Ethics Committee of Galicia (code 2022/480) and the rest of the participating centres.
PatientsInclusion CriteriaAdults with severe uncontrolled asthma treated with the same biologic for at least 24 months were included. If switched to another biologic, only data on the first treatment were included. Therefore, patients who had previously received another biologic were not included, and if the investigator decided to discontinue or change the biologic with which the patient entered the study, the patient was censored at that date, always after 24 months. Among the patients included, the first one to start a biologic was in 2011 (omalizumab) and the last one in 2022. Data collection extended from February to July 2024. All patients signed informed consent.
Exclusion CriteriaSmoking history >10 pack-years, incomplete essential data [exacerbations in the previous 12 months, oral corticosteroids (OCS) use, asthma control test (ACT) and spirometry], COPD diagnosis, or severe conditions leading to functional limitations or shortened life expectancy. Prior biologic therapy or diseases that might need biological therapy were also excluded.
Definitions- -
Asthma: Diagnosed according to Spanish Guidelines 15: symptoms of wheezing, breathlessness, or cough plus a positive bronchodilator test (>12% and 200mL FEV1 increase after the inhalation of 200mcg of salbutamol) or a positive methacholine test (PC20 <4mg/mL) or FENO ≥40ppb.
- -
Severe asthma: According to ERS/ATS guidelines as requiring high-dose inhaled corticosteroids plus a second controller and/or systemic corticosteroids, or remaining uncontrolled despite therapy.16
- -
Exacerbations: Only severe exacerbations were considered, identified as clinical deterioration events requiring systemic corticosteroids (≥3 days) and/or hospitalization.17
Definitions of response and failure to biologics:
- -
Response: No severe exacerbations in the prior 12 months, ACT ≥20 and no need for maintenance OCS.
- -
Sustained response: No severe exacerbations, ACT ≥20 and no need for maintenance OCS, throughout the entire follow-up period.
- -
Early failure (EF): No response was achieved at 12 months.
- -
Late failure (LF): Response was achieved at 12 months, but it was lost (either development of a severe exacerbation, need for maintenance OCS or ACT <20) in one of the visits that followed the 12-month visit.
Data were collected at the index date (biologic initiation), and at 12, 24 months post-initiation; and, if available, at 36 months and at the last clinic visit. Further details of the procedure are in the supplementary material.
Statistical AnalysisAs an exploratory study, no sample size calculation was performed; however, an estimated 300 patients were deemed necessary to analyse response types. Missing data were not imputed. Continuous variables are described by mean±SD and median (25th percentile–75th percentile); categorical variables are described as number (%). Normal distribution of continuous variables was assessed using Shapiro–Wilk tests. Baseline characteristics of patients were compared between groups (response at 12 months, early failure, sustained response and late failure) using χ2 tests for categorial variables and t tests and U tests for parametric and nonparametric variables, respectively. The association between asthma characteristics that in univariate analyses were associated with failure (early and late) with p<.10 (data not shown) were furthermore included in two multivariable logistic regression models to examine their independent impact on the odds of suffering failure (early failure in one of them and late failure in the other). Variables that were part of the definition of response (exacerbations, OCS burden, and ACT) and, as a consequence, part of early and late failure definitions, were not included in these models. FEV1 values were taken at baseline in the case of early failure and at 12 months in the case of late failure (only post-bronchodilation values were collected; in cases where spirometry was performed with the usual bronchodilator treatment, the result was considered post-bronchodilation). To explore the impact of different biologics on the presence of early or late failure, we decided to group them into two classes: anti-IgE (omalizumab) and anti-IL-5 (mepolizumab, benralizumab, reslizumab). Logistic regression model coefficients were converted into odds ratios for ease of interpretation and comparison. Analyses were conducted with SPSS software (version 23.0; IBM, Armonk, NY). Statistical significance was set at p<.05.
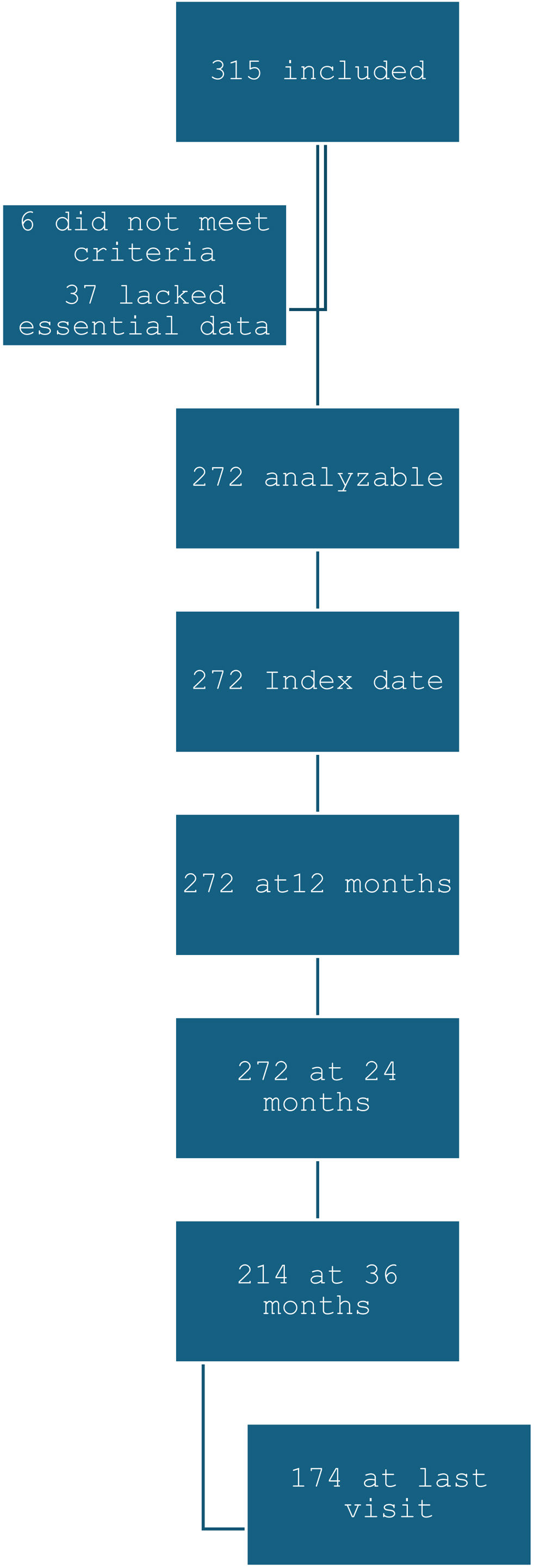
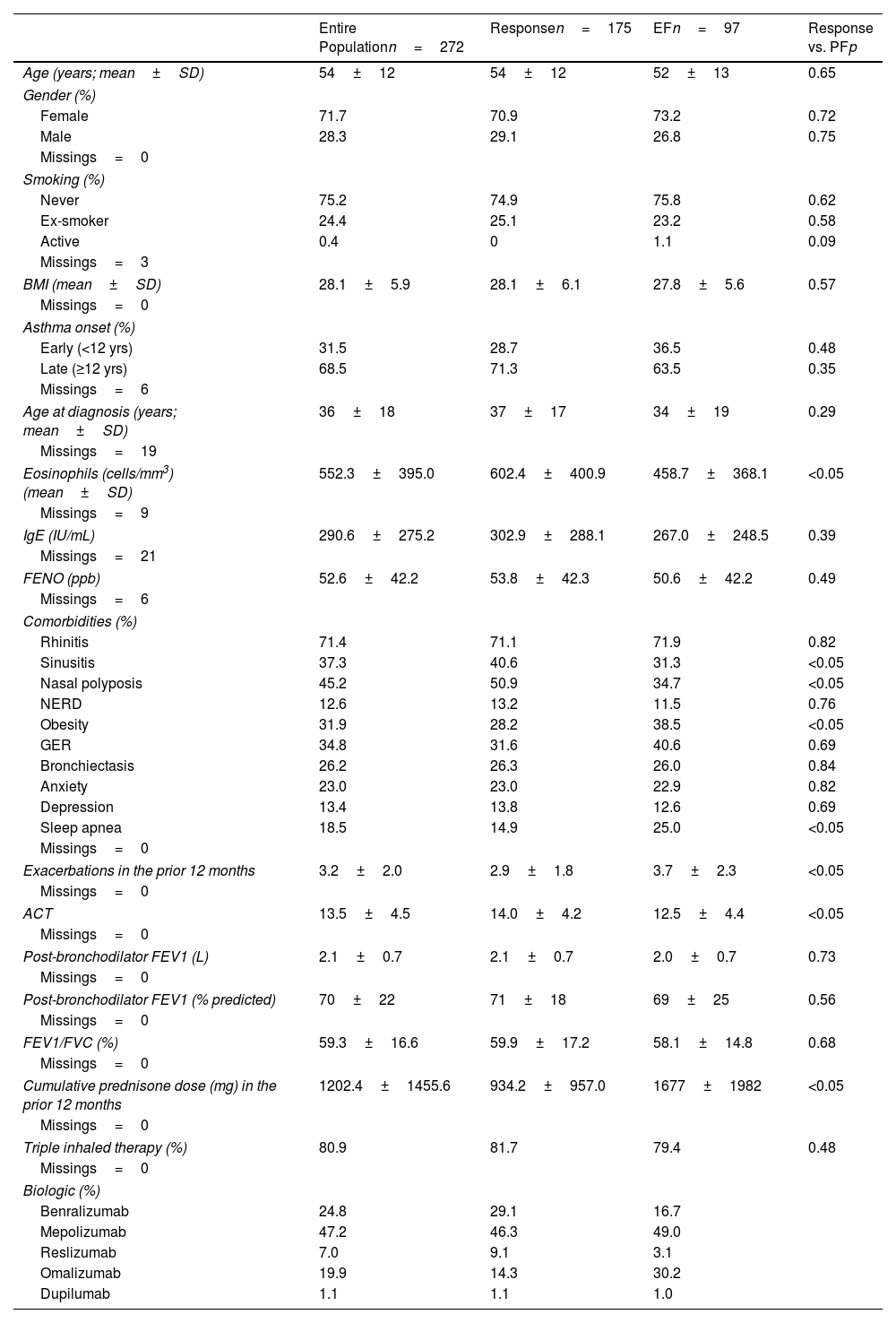
ResultsPopulationThree hundred fifteen patients were initially included; 6 did not meet eligibility criteria and 37 lacked essential data, resulting in 272 analyzable patients. Data were available at 12 and 24 months for all patients, at 36 months for 214, and at the final visit for 174 (Fig. 1). Mean follow-up was 46.1±19.4 months. Clinical and demographic characteristics are displayed in Table 1. Compared to responders at 12 months (175 patients), those with EF presented less clinical markers of T2 inflammation (nasal polyposis and chronic rhinosinusitis), lower eosinophil counts, more comorbidities and clinical features of greater severity.
Clinical and Demographic Characteristics of the Population. Comparison Between Patients who Experienced Response and Early Failure.
| Entire Populationn=272 | Responsen=175 | EFn=97 | Response vs. PFp | |
|---|---|---|---|---|
| Age (years; mean±SD) | 54±12 | 54±12 | 52±13 | 0.65 |
| Gender (%) | ||||
| Female | 71.7 | 70.9 | 73.2 | 0.72 |
| Male | 28.3 | 29.1 | 26.8 | 0.75 |
| Missings=0 | ||||
| Smoking (%) | ||||
| Never | 75.2 | 74.9 | 75.8 | 0.62 |
| Ex-smoker | 24.4 | 25.1 | 23.2 | 0.58 |
| Active | 0.4 | 0 | 1.1 | 0.09 |
| Missings=3 | ||||
| BMI (mean±SD) | 28.1±5.9 | 28.1±6.1 | 27.8±5.6 | 0.57 |
| Missings=0 | ||||
| Asthma onset (%) | ||||
| Early (<12 yrs) | 31.5 | 28.7 | 36.5 | 0.48 |
| Late (≥12 yrs) | 68.5 | 71.3 | 63.5 | 0.35 |
| Missings=6 | ||||
| Age at diagnosis (years; mean±SD) | 36±18 | 37±17 | 34±19 | 0.29 |
| Missings=19 | ||||
| Eosinophils (cells/mm3) (mean±SD) | 552.3±395.0 | 602.4±400.9 | 458.7±368.1 | <0.05 |
| Missings=9 | ||||
| IgE (IU/mL) | 290.6±275.2 | 302.9±288.1 | 267.0±248.5 | 0.39 |
| Missings=21 | ||||
| FENO (ppb) | 52.6±42.2 | 53.8±42.3 | 50.6±42.2 | 0.49 |
| Missings=6 | ||||
| Comorbidities (%) | ||||
| Rhinitis | 71.4 | 71.1 | 71.9 | 0.82 |
| Sinusitis | 37.3 | 40.6 | 31.3 | <0.05 |
| Nasal polyposis | 45.2 | 50.9 | 34.7 | <0.05 |
| NERD | 12.6 | 13.2 | 11.5 | 0.76 |
| Obesity | 31.9 | 28.2 | 38.5 | <0.05 |
| GER | 34.8 | 31.6 | 40.6 | 0.69 |
| Bronchiectasis | 26.2 | 26.3 | 26.0 | 0.84 |
| Anxiety | 23.0 | 23.0 | 22.9 | 0.82 |
| Depression | 13.4 | 13.8 | 12.6 | 0.69 |
| Sleep apnea | 18.5 | 14.9 | 25.0 | <0.05 |
| Missings=0 | ||||
| Exacerbations in the prior 12 months | 3.2±2.0 | 2.9±1.8 | 3.7±2.3 | <0.05 |
| Missings=0 | ||||
| ACT | 13.5±4.5 | 14.0±4.2 | 12.5±4.4 | <0.05 |
| Missings=0 | ||||
| Post-bronchodilator FEV1 (L) | 2.1±0.7 | 2.1±0.7 | 2.0±0.7 | 0.73 |
| Missings=0 | ||||
| Post-bronchodilator FEV1 (% predicted) | 70±22 | 71±18 | 69±25 | 0.56 |
| Missings=0 | ||||
| FEV1/FVC (%) | 59.3±16.6 | 59.9±17.2 | 58.1±14.8 | 0.68 |
| Missings=0 | ||||
| Cumulative prednisone dose (mg) in the prior 12 months | 1202.4±1455.6 | 934.2±957.0 | 1677±1982 | <0.05 |
| Missings=0 | ||||
| Triple inhaled therapy (%) | 80.9 | 81.7 | 79.4 | 0.48 |
| Missings=0 | ||||
| Biologic (%) | ||||
| Benralizumab | 24.8 | 29.1 | 16.7 | |
| Mepolizumab | 47.2 | 46.3 | 49.0 | |
| Reslizumab | 7.0 | 9.1 | 3.1 | |
| Omalizumab | 19.9 | 14.3 | 30.2 | |
| Dupilumab | 1.1 | 1.1 | 1.0 | |
BMI: body mass index; IgE: immunoglobulin E; FENO: fraction of exhaled nitric oxide; NERD: nonsteroidal antiinflammatory drug-exacerbated respiratory disease; GER: gastroesophageal reflux; ACT: asthma control test; FEV1: forced expiratory volume in the first second; FVC: forced vital capacity. Continuous variables with normal distribution were compared between the two groups using the independent samples t-test. Chi-square was used for categorical variables.
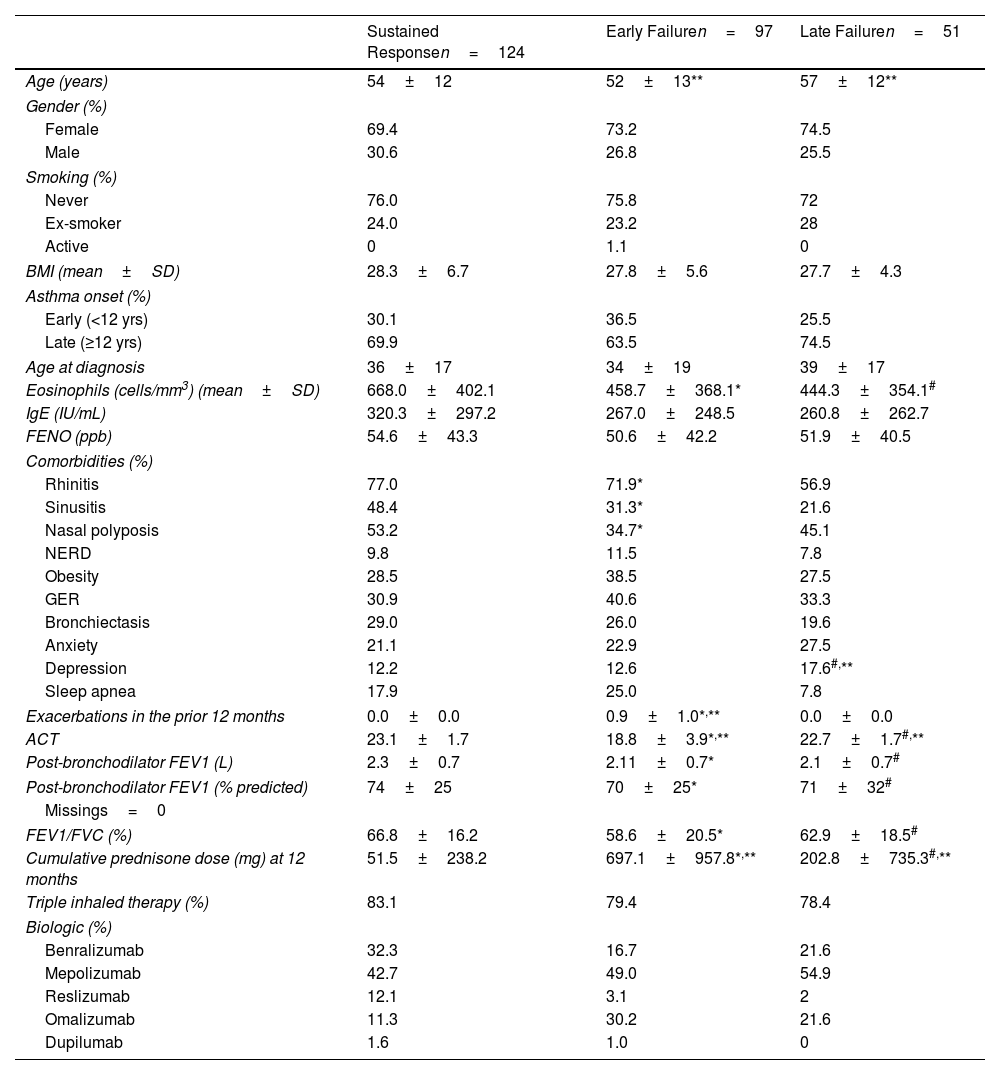
Among the 175 patients who attained response at 12 months, 124 (70.8%) experienced sustained response throughout the entire study period, while 51 (29.1%) showed LF at any of the subsequent visits. Compared to sustained response patients, those with LF had lower baseline eosinophil counts, were more frequently diagnosed with depression, achieved poorer lung function at 12 months and received a higher cumulative dose of corticosteroids during the first 12 months. The use of corticosteroids during the first months after biologic initiation in patients classified as responders is explained by their progressive reduction until discontinuation. These results are shown in Table 2.
Comparison Between Patients who Experienced Sustained Response and Early and Late Failure.
| Sustained Responsen=124 | Early Failuren=97 | Late Failuren=51 | |
|---|---|---|---|
| Age (years) | 54±12 | 52±13** | 57±12** |
| Gender (%) | |||
| Female | 69.4 | 73.2 | 74.5 |
| Male | 30.6 | 26.8 | 25.5 |
| Smoking (%) | |||
| Never | 76.0 | 75.8 | 72 |
| Ex-smoker | 24.0 | 23.2 | 28 |
| Active | 0 | 1.1 | 0 |
| BMI (mean±SD) | 28.3±6.7 | 27.8±5.6 | 27.7±4.3 |
| Asthma onset (%) | |||
| Early (<12 yrs) | 30.1 | 36.5 | 25.5 |
| Late (≥12 yrs) | 69.9 | 63.5 | 74.5 |
| Age at diagnosis | 36±17 | 34±19 | 39±17 |
| Eosinophils (cells/mm3) (mean±SD) | 668.0±402.1 | 458.7±368.1* | 444.3±354.1# |
| IgE (IU/mL) | 320.3±297.2 | 267.0±248.5 | 260.8±262.7 |
| FENO (ppb) | 54.6±43.3 | 50.6±42.2 | 51.9±40.5 |
| Comorbidities (%) | |||
| Rhinitis | 77.0 | 71.9* | 56.9 |
| Sinusitis | 48.4 | 31.3* | 21.6 |
| Nasal polyposis | 53.2 | 34.7* | 45.1 |
| NERD | 9.8 | 11.5 | 7.8 |
| Obesity | 28.5 | 38.5 | 27.5 |
| GER | 30.9 | 40.6 | 33.3 |
| Bronchiectasis | 29.0 | 26.0 | 19.6 |
| Anxiety | 21.1 | 22.9 | 27.5 |
| Depression | 12.2 | 12.6 | 17.6#,** |
| Sleep apnea | 17.9 | 25.0 | 7.8 |
| Exacerbations in the prior 12 months | 0.0±0.0 | 0.9±1.0*,** | 0.0±0.0 |
| ACT | 23.1±1.7 | 18.8±3.9*,** | 22.7±1.7#,** |
| Post-bronchodilator FEV1 (L) | 2.3±0.7 | 2.11±0.7* | 2.1±0.7# |
| Post-bronchodilator FEV1 (% predicted) | 74±25 | 70±25* | 71±32# |
| Missings=0 | |||
| FEV1/FVC (%) | 66.8±16.2 | 58.6±20.5* | 62.9±18.5# |
| Cumulative prednisone dose (mg) at 12 months | 51.5±238.2 | 697.1±957.8*,** | 202.8±735.3#,** |
| Triple inhaled therapy (%) | 83.1 | 79.4 | 78.4 |
| Biologic (%) | |||
| Benralizumab | 32.3 | 16.7 | 21.6 |
| Mepolizumab | 42.7 | 49.0 | 54.9 |
| Reslizumab | 12.1 | 3.1 | 2 |
| Omalizumab | 11.3 | 30.2 | 21.6 |
| Dupilumab | 1.6 | 1.0 | 0 |
Comparisons are made at baseline for demographic characteristics and biomarkers (FENO, eosinophils) and at 12 months for response variables (exacerbations, ACT, corticosteroid load, and spirometry). BMI: body mass index; IgE: immunoglobulin E; FENO: fraction of exhaled nitric oxide; NERD: nonsteroidal antiinflammatory drug-exacerbated respiratory disease; GER: gastroesophageal reflux; ACT: asthma control test; FEV1: forced expiratory volume in the first second; FVC: forced vital capacity. Continuous variables with normal distribution were compared between groups using the independent samples t-test. Chi-square was used for categorical variables.
Ninety-seven patients were classified as EF at 12 months. On multivariable analysis, lower eosinophil counts (OR 2.489, 95%CI 1.837–3.942; p=0.04), sleep apnea diagnosis (OR 1.909, 95%CI 1.037–2.942; p=0.03) and absence of nasal polyps (OR 2.265, 95%CI 1.375–4.146; p=0.03) were independently associated with greater likelihood of EF. EF reverted in 40% of patients at later visits without switching biologics (by changes in inhaled corticosteroid/β2-agonist combination or adding long-acting anticholinergic in 74%; Table S1 and Fig. 2). Severe exacerbations coupled with ACT <20 were the most common clinical manifestations of EF (Table S2). Interestingly, of the 45 patients (33.3%) who did not achieve two response domains (mostly ACT+exacerbations), only 15 patients achieved response at subsequent visits, with 11 adjusting inhaled therapy.
Patients With Late FailureFifty-one out of 175 patients (29.1%) who responded at 12 months experienced LF at later visits. On multivariable analysis, lower baseline eosinophil counts (OR 1.6, 95%CI 1.132–2.146; p=0.03), and lower FEV1 at 12 months (OR 1.989, 95%CI 1.279–2.896; p=0.02) were independently associated with greater likelihood of LF. Severe exacerbations were the most common manifestation (Table S3). LF reverted (response was recovered) in 9 of 25 patients (36%) where subsequent follow-up visits were available (41.6% after adjusting inhaled therapy) (Fig. 2). Among the manifestations of failure, response recovery was observed in 3 of 7 patients with ACT <20 and in 5 of 16 patients with exacerbations. FEV1 decreased by ≥100mL from the value at the previous visit in 12 of 16 cases that did not recover response after LF (Table S4). Among 14 patients with just one exacerbation, only 5 recovered response at the subsequent visit.
DiscussionThis real-world study, involving patients on the same biologic for an extended period (only with this design could we address the concepts of sustained response and LF), showed that 70.8% of those who achieved response at 12 months maintained it throughout the entire study period. Response was defined as having no severe exacerbations within 12 months, controlled symptoms and no systemic corticosteroid use. The definition of biological response is not unanimously accepted, but the trend is towards the adoption of more stringent criteria,18,19 especially with regard to exacerbations and the use of OCS. Lung function was excluded from our response definition, despite being a common component in clinical response or remission criteria,18,19 because in clinical practice, a certain degree of bronchial obstruction can be considered acceptable if all other therapeutic objectives are achieved. In addition, in most cases it is not possible to know in advance how much lung function can be improved with a biologic. Classically, it was believed that remodeling is irreversible, but recent studies have shown that it can be at least partially reversed.20 For these reasons, it is difficult to know whether, when a biologic is started, the best possible response in pulmonary function has been achieved. We found that 29% of patients who responded at 12 months lost response over time. These patients, classified as LF, had lower baseline eosinophil counts and were more often diagnosed with depression compared to those with sustained response. Patients who lost response during follow-up had lower FEV1 values at 12 months, underlining the importance of lung function in the response to biologics, as noted in previous studies.6 We acknowledge that the concept of LF that we have used in this study is questionable; this is the first time it has been defined in SA. We have decided that the ultimate goal of asthma management – aligned with that of international guidelines15 – is, at a minimum, to keep the patient free of severe exacerbations and with well-controlled symptoms, and the definition of LF agreed upon in this study is consistent with this.
Remarkably, approximately 40% of patients with EF and 36% with LF recovered response at a subsequent visit, either without treatment changes or by modifying inhaled therapy. EF occurred, in most cases, when two of the components of the response definition were not met, and response recovery was rare unless inhaled therapy was adjusted. It was an unexpected finding that, in clinical practice, some physicians decided to change inhaled medication instead of switching the biologic. It should be kept in mind that inhaled corticosteroids have different pharmacological properties: receptor affinity, receptor binding time, lipophilicity, therapeutic index, and others.21 In addition, it is well known that notable between-device differences in release mechanism, particle size, drug deposition and required inspiratory flow exist,22 so changes in the inhalation device may also have had an impact on the results. One of the key messages of this study is that inhaled therapy should be optimized before starting a biologic and should also be reconsidered in cases of EF and LF, before deciding to switch to another biologic drug. This could be helpful in preserving a therapeutic target and avoiding unnecessary changes that may pose a risk to the patient.
No real-world studies are available to estimate the frequency and persistence of SF to biologics, as the usual practice is to switch biologics when patients worsen. Thus, this study provides data that are difficult to obtain. A post hoc analysis of the QUEST and TRAVERSE studies indicated that 70.2% of patients meeting all criteria for asthma remission in Year 1 continued to meet them in Year 2,23 indirectly suggesting that 30% of patients worsened over time. A similar percentage (68%) of sustained response at 36 months was found by Pini et al. in a study involving 108 patients treated with benralizumab.24 Noteworthily, these percentages are almost the same as those in our study. Another study that used the same definition of response as ours and analyzed 303 patients with SA who received mepolizumab, found that 43.2% achieved it after 12 months and 29.7% remained in “sustained remission” during 24 months,25 suggesting that some patients may develop LF and that the response is variable over time.
EF and LF could share certain causes of failure (e.g., comorbidities limiting improvement, insufficient dosage, autoimmune phenomena, or infections).26 Nevertheless, it is plausible that other causes differ. Incorrect identification of the specific T2 pathways in a patient's asthma may cause EF, while changes in the inflammatory endotype and anti-drug antibody development may contribute to LF. Anti-drug antibody incidence has been reported as approximately 8% for benralizumab and dupilumab, and less than 5% for other biologics,27 although few studies have addressed its impact on biological therapy efficacy and safety.
While there is some consensus on defining EF or suboptimal response, LF has not been characterized, which complicates clinical decision-making about maintaining or switching biologics. After 12 months of biologic therapy, poorer lung function was associated with LF, suggesting clinicians should monitor these patients more closely. Deciding whether to switch biologics after a single exacerbation – often viral-triggered – is particularly challenging. We know that most exacerbations in patients with severe asthma are viral in origin,28 and also that biologics can prevent them in about half of the cases.5 However, studies are needed to establish whether a virus-triggered exacerbation is a one-off event or will recur over time. Our study found that only 5 out of 16 patients with a single exacerbation recovered response at subsequent visits, and an FEV1 decrease of ≥100mL made subsequent recovery unlikely.
One of the strengths of this study is introducing a novel concept of biological response in asthma. We believe differentiating EF and LF may help unravel the mechanisms leading to suboptimal response. Also noteworthy is its multicenter design and the long follow-up period with the same biologic. However, limitations include the retrospective database methodology, although we have tried to minimize the impact of missing data by including only those cases where essential data were available.
Another limitation is that the follow-up period was not the same for all patients, and, in some cases, we could not obtain data from a follow-up visit after an LF was identified. Moreover, although the follow-up was long (46.1±19.4 months), extending it further might reveal a higher LF rate. It should also be noted that many patients were treated with omalizumab, a biologic less frequently used today, but its inclusion allowed us to study the behavior of cases where the biologic was maintained despite suboptimal response. The design of this study (which requires maintaining the same biologic for at least 24 months regardless of whether the response had been suboptimal, something that does not occur in current clinical practice) does not allow comparisons of effectiveness between biologics. However, we did not find that using a particular class of biologics (anti-IgE or anti-IL-5) was associated with a higher likelihood of failure. We acknowledge that it would be very interesting to know whether LF occurs more frequently with one class of biologics (or with a specific biologic) than with others, but we believe that we do not have sufficient statistical power to answer this question with confidence. Perhaps data from international registries with large numbers of patients could be helpful.
In conclusion, this study showed that among SA patients treated with biologics, 78.9% of those who achieved a response at 12 months maintained it long-term. Approximately 40% of patients with EF or LF recovered response at a subsequent visit, either with no change in treatment or, more frequently, with changes in inhaled medication. An FEV1 decrease of at least 100mL predicted non-recovery of response at subsequent visits. Further studies are needed to corroborate our findings and to explore the mechanisms underlying EF and LF in greater depth in order to increase the percentage of patients who achieve a complete and sustained response over time.
CRediT Authorship Contribution StatementLuis Pérez de Llano agrees to be accountable for all content and aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. David Dacal and Luis Pérez de Llano had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors were involved in data acquisition or analysis and interpretation, as well as the critical revision of the manuscript for important intellectual content. All authors were involved in the conception and design of the study. All authors were responsible for drafting the manuscript. All authors provided additional administrative, technical, and material support. The study was supervised by Luis Pérez de Llano and David Dacal. All authors approved the final version of this manuscript and agreed to be accountable for all aspects of the work.
Declaration of Generative AI and AI-assisted Technologies in the Writing ProcessThe authors declare that no part of the material in this manuscript has been partially or totally produced with the assistance of any artificial intelligence software or tool.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of InterestDr. Pérez de Llano reports grants, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from GSK, grants and personal fees from TEVA, personal fees and non-financial support from Novartis, personal fees and non-financial support from Chiesi, personal fees from Sanofi, personal fees from Menarini, grants and personal fees from Esteve, personal fees from MSD, personal fees from TECHDOW PHARMA, grants and non-financial support from FAES, personal fees from Leo-Pharma, personal fees from GEBRO, personal fees from GILEAD, outside the submitted work. Eva Martinez-Moragón in the last three years has received honoraria for speaking at sponsored meetings from AstraZeneca, Chiesi, GSK, Gebro, Menarini, and Sanofi; and has acted as a consultant for AstraZeneca, Chiesi, GSK, and Menarini. Carolina Cisneros in the last three years has received honoraria for speaking at sponsored meetings from AstraZeneca, Chiesi, GSK, Gebro, Menarini, and Sanofi; and has acted as a consultant for AstraZeneca, GSK, Sanofi and CIPLA. Hemily K. Izaguirre Flores reports grants, personal fees and non-financial support from Sanofi, grants, personal fees and non-financial support from GSK, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Chiesi, non-financial support from GEBRO, personal fees from Menarini, outside the submitted work. VP in the last three years has received honoraria for speaking at sponsored meetings from AstraZeneca, Boehringer-Ingelheim, Chiesi, Gebro, GSK, Luminova-Medwell, and Sanofi; has received assistance for travel from AstraZeneca and Chiesi; and has acted as a consultant for AstraZeneca, Chiesi, GSK, and Menarini. Cleofé Fernández reports personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from GSK, personal fees and non-financial support from Chiesi, personal fees and non-financial support from Sanofi, personal fees from Menarini, and personal fees and non-financial support from GEBRO, outside the submitted work. Silvia Sánchez-Cuéllar has received, in the last 5 years, honoraria for: speaking at meetings sponsored by AstraZeneca, GSK, Chiesi, Faes, Gebro Pharma, Novartis; attendance at congresses and scientific meetings by AstraZeneca, Bial, Chiesi, GSK, Sanofi, Novartis and Gebro Pharma, for acting as a consultant for AstraZeneca, GSK, Novartis and Sanofi and for research projects by AstraZeneca, GSK, Sanofi and Novartis. Dr. Serrano Rebollo reports personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from GSK, personal fees from Chiesi, personal fees from Sanofi, non-financial support from Menarini, personal fees from FAES, personal fees from GEBRO, outside the submitted work. Borja G. Cosio declares grants from Chiesi and GlaxoSmithKline; personal fees for advisory board activities from Chiesi, GlaxoSmithKline, Novartis, Sanofi, Teva, and AstraZeneca; and payment for lectures/speaking engagements from Chiesi, Novartis, GlaxoSmithKline, Menarini, and AstraZeneca, outside the submitted work. Dr. Dacal Rivas reports personal fees and non-financial support from Esteve, GSK, Novartis, TEVA, Chiesi, Ferrer, FAES Farma, AstraZeneca and Cipla outside the submitted work. In the last three years, Dr. Dávila has received payment for lectures, including service on speaker's bureaus from Allergy Therapeutics, ALK, AstraZeneca, Chiesi, Diater, GSK, Leti, Novartis, Sanofi; for a consultancy from Allergy Therapeutics, ALK-Abello, AstraZeneca, GSK, Merck, MSD, Novartis, Sanofi; and grants for Thermofisher Diagnostics, ISCIII and Junta de Castilla y León.
The authors thank PInvestiga for their support in developing the electronic record system.
Daniel Castillo Otero (Hospital Universitario Puerto Real). Carlos Sabadell Nieto (Hospital de Figueres). Anna Michela Gaeta (Hospital Universitario Severo Ochoa). David Díaz Pérez (Hospital Universitario Nuestra Señora de la Candelaria).