Cerebrovascular disease has emerged as one of the main causes of morbidity and mortality worldwide. Having a stroke doubles the risk of developing dementia in the future, and recurrent events raise the prevalence of dementia to 30%.1 The relationship between hypertension and both brain structure and function is only partially known. Current evidence suggests that several pathological factors can influence it. Hypertension can produce vascular and functional changes in larger and small cerebral vessels in the brain. Moreover, cerebral small vessels and arterioles are more vulnerable to the mechanical stress associated with hypertension.2 In addition, chronic hypertension induces a rightward shift in the autoregulatory curve, and consequently, can increase vulnerability to sudden changes in blood pressure, resulting in ischemia or increased risk of brain hemorrhage.2,3 On the other hand, recent evidence suggests that acute and chronic hypertension could produce blood-brain barrier damage, which is crucial for blood pressure regulation. Brain sympathetic nervous system activation and brain–renin–angiotensin activation, associated with endothelial injury, oxidative stress and inflammation are involved in the breakdown of blood–brain barrier.4
Cerebral small vessel disease (CSVD) refers to a syndrome of clinical and neuroimaging findings resulting from pathologies in the small perforating cerebral arterioles, capillaries, and venules, manifested on magnetic resonance imaging (MRI) or pathology examination. Clinically, CVDS presents as ischemic and hemorrhagic stroke, and it is responsible of 25% of ischemic stroke (lacunar strokes), a higher percentage of hemorrhagic strokes and for up to 45% of dementias either as vascular or mixed with Alzheimer disease.2–7
Pathology studies describe abnormalities in arterioles, such as arteriosclerosis, lipohyalinosis, or necrosis fibrinoide and the pathologic findings suggest that the pathogenesis of CSVD is more complex than only arteriolar occlusion that leads to infarcts.2 The characteristic findings of CSVD in RMI include lacunar infarcts, white matter hyperintensities (WMH) cerebral microbleeds (CMB), enlarged perivascular spaces, and brain atrophy.8,9 Its prevalence is about 5% in subjects over 50 and it is present in almost everyone by the age of 90.4
CSVD could be usually attributed to classical risk factors, specifically to hypertension, diabetes and smoking, but of them, hypertension represents a key factor in the development of CSVD. Endothelial dysfunction is considered to appear at an early stage of CSVD, before any radiologic signs evidence, and although it can be measured by functional transcranial Doppler, this technique has major limitations in terms of operator variability.8
Obstructive sleep apnoea (OSA) is a known risk factor of several cardiovascular diseases such as arrhythmia, coronary head disease or stroke, and is an independent cause of resistant hypertension.10,11 In addition, patients with refractory hypertension (subjects that remain uncontrolled hypertension despite the administration of at least 5 antihypertensive drugs),12 have an even greater prevalence and severity of OSA than resistant hypertension patients.13,14
The relationship of OSA with CSVD is less well known, and in the last few years several studies have started to explore the relationship of OSA and CSVD by neuroimaging findings. In a systematic review and meta-analysis, Chokesuwattanaskul et al.,15 have shown an association between OSA and CSVD by MRI findings of WMH and covert lacunar infarction. When compared to patients without OSA, this study demonstrates that OSA is an independent risk factor of asymptomatic CSVD. A more recent meta-analysis, including thirty-two observational studies,16 have reported that compared to patients without OSA, the odds ratio of WMH were 3.9 (CI 95% 2.7–5.5) in subjects with moderate-severe OSA and 4.3 (CI 95% 1.9–9.6) in severe OSA. In contrast, OSA had no association with cerebral microbleeds and an undetermined association with both perivascular spaces and subcortical infarcts due to insufficient data.
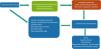
The possible mechanisms responsible for the present relationship of OSA with CSVD could include vascular endothelial dysfunction, hypoxia, hypoperfusion, reperfusion, oxidative stress, neuroinflammation and hypercoagulability state. In addition, OSA is associated with sympathetic activation, which is detectable even in early stages of its evolution and in the absence of comorbidities or other cardiometabolic diseases. This activation is directly proportional to the frequency of apnoeas and hypopneas during sleep.17 In chronic untreated OSA, sympathetic activation during wakefulness could be attributed to long-term structural or functional neural adaptations to hypoxia and oxygen free radical generation due to recurring apnoeas and hypopneas during sleep.18 In Fig. 1, we show the possible mechanisms involved in the relation between OSA, hypertension and cerebral small vessel disease.
On the other hand, OSA has been associated with resistant or refractory hypertension, and has shown a high prevalence of uncontrolled nocturnal hypertension, possibly one of the causes of the worse functional prognosis of cerebrovascular disease in these patients. It is very well known that abnormal dipping patterns may affect vascular health independent of BP level, and several studies have shown that higher night-time BP levels and a riser pattern are independently associated with the total cardiovascular event rate.19 Elevated night-time BP and reverse-dipping compared with normal dipping BP pattern have been associated with subclinical cerebrovascular disease.20,21
Hypertension is the most important modifiable risk factor to prevent stroke, and it is strongly associated with the development of CSVD. Therefore, the primary prevention of stroke and its recurrences is a priority strategy for the prevention of dementia. Despite the deleterious effects of hypertension, BP control remains suboptimal: less than 50% of adults achieve adequate BP control. These data have worsened in recent years, probably due to low adherence and early discontinuation of antihypertensive treatment. In addition, it is known that OSA is associated with resistant and uncontrolled nocturnal hypertension.
Primary and secondary prevention of stroke and CSVD is a priority for the prevention of dementia. Mild cognitive impairment (MCI) is often considered as an intermediate phase between normal cognition and dementia, common in patients with hypertension. MCI has an overall prevalence of 11.8–30% and it increases the likelihood of progression to dementia or death compared with having no cognitive impairment. Suvila et al.22 also observed that early- but not late-onset hypertension is related to midlife cognitive function. In addition, among patients with OSA and resistant hypertension, CPAP treatment for 12 weeks compared with control, resulted in a reduction in 24h BP and an improvement in nocturnal BP or circadian BP pattern.23 On the other hand, recent meta-analysis has shown that adherence to CPAP was associated with a reduced or major cardiovascular events recurrence risk.24
In conclusion, OSA is highly prevalent in hypertension patients, especially in resistant or refractory hypertension. Although OSA increases the risk of all-cause and cardiovascular mortality, this condition is often underrecognized and undertreated in cardiovascular practice. We believe that early improvement in hypertension control among middle-aged hypertensive individuals, coupled with ensuring adequate adherence to antihypertensive treatment and CPAP therapy in patients with hypertension and OSA, are essential components for preventing cardiovascular events and the onset of early cerebrovascular disease and cognitive decline.
AcknowledgeThe authors would like to express their gratitude to Mariana Ubeda-Romero Araujo for reviewing the manuscript.
Conflict of interestsThe authors state that they have no conflict of interests.