Pulmonary hypertension (PH) is a frequent but often unrecognised complication of chronic lung diseases (CLD), including chronic obstructive pulmonary disease (COPD), interstitial lung disease (ILD) and obesity-hypoventilation syndrome (OHS), among the most common causes [1]. Diagnosing PH associated with CLD (PH-CLD) remains particularly challenging due to the heterogeneity of its clinical manifestations and the absence of standardised diagnostic approach [2].
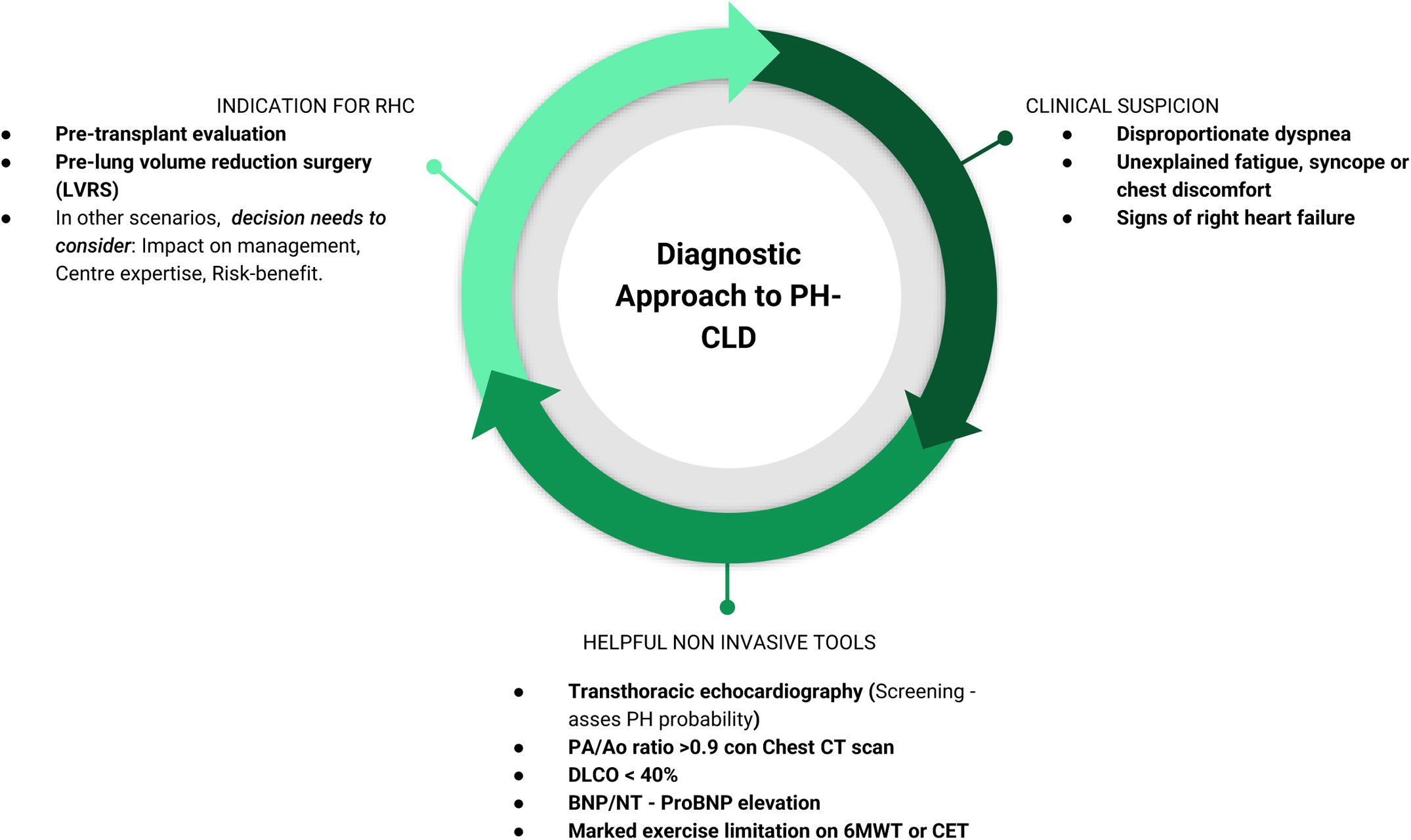
In the current issue of Archivos de Bronconeumologia, Rodríguez-Chiaradía et al. present a noteworthy document of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR), focusing on the diagnosis of PH-CLD in the setting of COPD, ILD and OHS [3]. This contribution deserves commendation for its attempt to bring clarity to a long-standing unresolved question. Building on a comprehensive systematic review of the literature and experts’ opinion, the primary objective of the document is to provide a practical diagnostic framework that can assist clinicians in identifying PH-CLD and guide clinical decision-making to the benefit of both patients and healthcare systems. The authors provide diagnostic algorithms structured around three pivotal questions: (1) when to suspect PH, (2) what tests should be used to detect it, and (3) when right heart catheterisation (RHC) is indicated (Fig. 1). While these algorithms represent a thoughtful and practical approach, they should be viewed as proposals requiring prospective validation. Moreover, it is important to mention that the quality of the evidence to support these recommendations has room for improvement, underscoring the urgent need for further research in this domain.
Diagnostic stepwise approach to PH-CLD. RHC: right heart catheterisation; PA/AO ratio: pulmonary artery/aortic ratio; Chest CT: chest computerized tomography; DLCO: diffusing capacity of the lungs for carbon monoxide; BNP: B-type natriuretic peptide; NT-proBNP: N-terminal pro-B-type natriuretic peptide; 6MWT: 6 minutes walking test; CET: cardiopulmonary exercise test.
The proposed stepwise diagnostic strategy in PH-CLD begins with a careful clinical assessment. An important clinical indicator is the presence of a discrepancy between the severity of symptoms, particularly dyspnoea, and the extent of parenchymal lung disease as assessed by pulmonary function tests or thoracic imaging. Although such discrepancies may be due to other comorbidities, the possibility of PH must be actively considered. Transthoracic echocardiography (TTE) is a central tool in this context, for assessing the likelihood of PH. The echocardiographic probability of PH is primarily based on the estimation of systolic pulmonary artery pressure using tricuspid regurgitation velocity (when measurable). However, it should also incorporate indirect signs suggestive of elevated pulmonary pressures, such as right atrial or right ventricular enlargement, interventricular septal flattening, or evidence of right ventricular dysfunction [4]. Additional supportive findings that may raise suspicion for PH include elevated natriuretic peptides, a pulmonary artery-to-aorta diameter ratio >0.9 on a chest computed tomography (CT), a diffusion lung capacity for carbon monoxide (DLCO) <40%, and marked exercise limitation (six-minute walk distance or cardiopulmonary exercise testing) [5]. Once PH is suspected, RHC remains the gold standard for confirmation. However, unlike in pulmonary arterial hypertension (PAH), the decision to perform RHC in PH-CLD should not rely solely on echocardiographic probability but must also take into account whether the results will impact clinical management or therapeutic decisions. Beyond this, the SEPAR document strongly recommends RHC in two specific contexts: (1) candidates for lung transplantation, and (2) patients with COPD undergoing evaluation for lung volume reduction surgery. Thus, the key clinical challenge is not merely detecting PH in CLD but identifying the subset of patients in whom PH significantly alters prognosis or requires a tailored therapeutic approach.
Several studies have demonstrated the importance of the presence and severity of PH in the outcomes of CLD [6,7]. Likewise, the haemodynamic definition of PH, as well as the criteria for assessing PH severity in CLD have been updated in the recent 2022 ESC/ERS guidelines [4]. In this sense, Mora Cuesta et al. retrospectively evaluated a cohort of patients with advanced respiratory disease undergoing RHC for lung transplant assessment [8]. When applying the 2022 PH definition to this cohort of PH group 3 compared to the 2015 definition, the proportion of patients meeting PH-CLD criteria increased significantly, but with a lower proportion of severe PH (60.7% by 2015 definition vs 8.4% by 2022 definition). This shift has practical implications and reinforces the necessity of individualised decision-making regarding invasive procedures such as RHC, which should ideally be performed in expert centres and limited to patients who are likely to derive a clear clinical benefit.
Although treatment options remain limited and lack strong evidence of their benefit [9,10], it is important to highlight recent positive results of the INCREASE trial on the specific subset of patients with PH associated ILD which makes the evaluation of these patients even more important [11].
Given the increasing sophistication of risk stratification in PAH, using multiparametric assessment that includes clinical, laboratory, functional, and imaging parameters, it would be reasonable to apply the same approach to PH-CLD [12]. Such an approach could greatly enhance both prognostic accuracy and treatment individualisation. Importantly, the underlying lung disease significantly influences prognosis and therapeutic response, making it crucial that any prognostic or therapeutic models integrate disease-specific variables. Finally, it is important to recognise that chronic respiratory diseases differ significantly from one another, each with a distinct natural history and potentially divergent response to PAH-approved therapies. This heterogeneity calls for the development of disease-specific assessment tools, an ambitious but essential endeavour that aligns perfectly with the principles of personalised medicine.
In conclusion, as López Meseguer et al. also pointed out in this journal recently, we are only beginning to understand how PH behaves when it coexists with lung disease [13]. In the upcoming years, we expect significant advances in knowledge in this area that will contribute to establish more robust recommendations. Pending further evidence, this SEPAR document stands as a significant and pragmatic contribution, serving both as a call to awareness and as a practical tool to support the diagnostic approach to PH-CLD.
CRediT authorship contribution statementDC: Writing first draft. All authors: Writing, review and editing. All authors have read and agreed with the final version of the manuscript.
Ethical statementNo concerns as there are no patients or data involved in this manuscript.
Declaration of generative AI and AI-assisted technologies in the writing processWe certify that this manuscript was not generated using any artificial intelligence tools.
FundingThis research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestDC declares having received grants and personal fees from Boehringer-Ingelheim, personal fees from Veracyte, and grants from Fujirebio, all outside the submitted work. DM declares having received grants from Gossamer and Merck, consulting fees from Ferrer, Merck MSD, and honoraria for lectures or educational events from Bayer, Boehringer, Chiesi, GSK, Jazz pharmaceuticals, Ferrer and Merck MSD, all outside the submitted work. JF and MP declare no conflicts of interest.