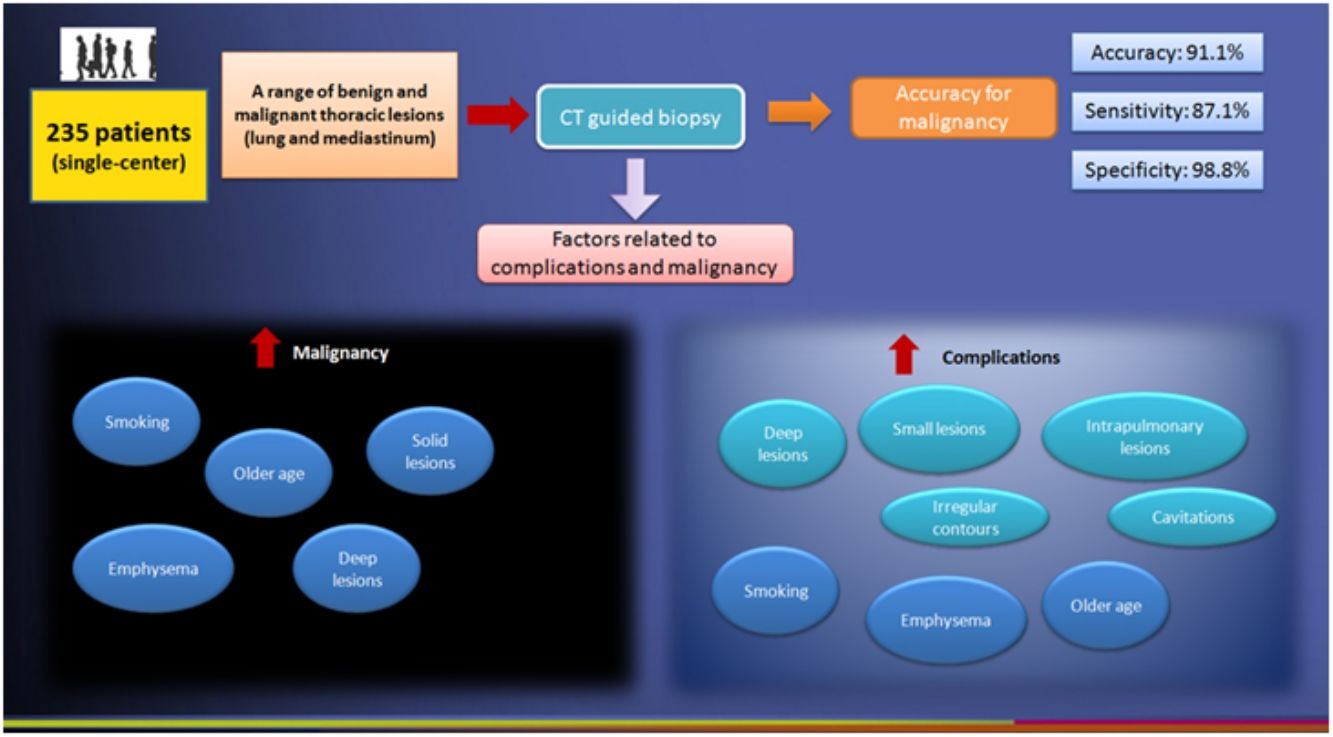
To assess the impact of patient-, lesion- and procedure-related factors on the risks of complications and final diagnosis of malignancy in PCNB of mediastinal and lung lesions.
Material and MethodsWe studied a large single-center cohort of 235 consecutive patients (66.8% men; 58.5±18.0 years) with a range of thoracic benign and malignant lesions, who underwent PCNB performed along 24 months by a single experienced radiologist. Diagnostic accuracy analyses of PCNB for malignancy were performed, as well as estimations of relative risk and logistic regression models in order to assess possible associations between such factors and malignancy/complications.
Results155 lesions (65.9%) were diagnosed as malignant. Overall accuracy was 91.1%, with sensitivity of 87.1%, specificity of 98.8%, positive predictive value of 99.3%, and negative predictive value of 79.8%. Pneumothorax (49/235; 20.8%) and hemorrhage (37/235; 15.7%) were the most common complications. Emphysema, smoking, older age, intrapulmonary location, deeper location, smaller size, presence of cavitations and irregular contours of the lesions, and smaller needle-pleural angles were the most consistent factors related to the occurrence of complications. Emphysema, older age, smoking, solid and deeper lesions were also significantly associated with a final diagnosis of malignancy after PCNB.
ConclusionCT-guided PCNB of mediastinal and lung lesions is a safe procedure with high diagnostic accuracy for malignancy.
Evaluar el impacto de los factores relacionados con el paciente, la lesión y el procedimiento en el riesgo de complicaciones y que el diagnóstico final sea cáncer en la biopsia percutánea con aguja gruesa (B.A.G) de lesiones mediastínicas y pulmonares.
Materiales y métodosSe estudió una cohorte unicéntrica grande de 235 pacientes consecutivos (66,8% hombres; 58,5±18,0 años) con diversas lesiones torácicas benignas y malignas, a los que un único radiólogo experimentado practicó B.A.G. a lo largo de 24 meses. Se realizaron análisis de precisión diagnóstica del B.A.G para el cáncer, así como estimaciones del riesgo relativo y modelos de regresión logística para evaluar las posibles asociaciones entre esos factores y diagnóstico de cáncer y/o aparición de complicaciones.
ResultadosSe determinó que 155 lesiones (65,9%) eran malignas. La precisión total fue del 91,1%, con una sensibilidad del 87,1%, una especificidad del 98,8%, un valor predictivo positivo del 99,3% y un valor predictivo negativo del 79,8%. Las complicaciones más frecuentes fueron el neumotórax (49/235; 20,8%) y la hemorragia (37/235; 15,7%). Los factores relacionados de forma más consistente con la existencia de complicaciones fueron el enfisema, el tabaquismo, la edad avanzada, la localización intrapulmonar, la localización profunda, el tamaño pequeño, la presencia de lesiones cavitadas con contornos irregulares y los ángulos aguja-pleura más pequeños. El enfisema, la edad avanzada, el tabaquismo y las lesiones sólidas y profundas también se asociaron significativamente con un diagnóstico final de cáncer después de la B.A.G.
ConclusiónLa B.A.G guiada por TC de lesiones mediastínicas y pulmonares es un procedimiento seguro con una elevada precisión diagnóstica para la detección del cáncer.
Computed tomography (CT)-guided transthoracic needle biopsy (CT-TNB) is a common and well-established procedure in the histological diagnosis of pulmonary and mediastinal lesions.1–4 Owing to inherent technical advances and its better detection of thoracic lesions (some of which cannot be identified on chest radiograph), CT has rapidly become the modality of choice for performing transthoracic needle biopsies, with reported diagnostic accuracy rates ranging from 64% to 97%.1,5,6
CT-TNB can be performed in two ways: (a) CT-guided percutaneous core needle biopsy (PCNB) uses a core needle or cutting needle to obtain a specimen for histological examination; (b) percutaneous fine-needle aspiration biopsy (PNAB) provides a cytological specimen.7,8 However, many of current studies are inhomogeneous in terms of utilization of PCNB or PNAB.7,9 Recently, a meta-analysis demonstrated that, although the diagnostic accuracy differences between such techniques are not obvious, the current literature is affected by publication biases that prevent confident conclusions,7 emphasizing the need for further and methodologically better studies performed with more homogeneous characterization in terms of sample and type of technique.
Moreover, as most studies have been strictly focusing only on pulmonary nodules,7,10 they do not tend to reflect appropriately the daily clinical practice faced by the interventional radiologist, in which, beyond pulmonary nodules and masses, a range of mediastinal and pleural lesions must also be assessed,9,11,12 reinforcing the importance of studies addressing a myriad of lesions.
Additionally, it is important to point out that pneumothorax and perifocal hemorrhage remain the most frequent complications of CT-TNB and chest tube placement for treatment of pneumothorax may be required sometimes.9,13–15 Reported pneumothorax rates in CT-TNB vary largely (range from 8% to 69% in some studies), depending, for example, on location and size of the lesion, and experience of the interventional radiologist.9,13,16 However, the literature has also been inconsistent and conflicting in terms of factors (e.g., lesion size, length of biopsy path and number of pleural needle passes) affecting complication rates.9,15–17 Indeed, most of such studies are affected by another bias, which is related to performer's experience and the employment of a heterogeneous range of performers,9,15–17 and a single-operator strategy might be an option to overcome these between-radiologist heterogeneity limitations.18 Finally, some studies have included relatively small sample sizes (even with less than 30 patients), which highlights the need for large samples.
Because complication rates and diagnostic performance are the two main factors in choosing a diagnostic procedure7 and owing to all the current limitations listed above, the present study was conducted in order to assess the impact of technical factors, lesion features and patient-related characteristics on the risks for a final diagnosis of malignancy and of procedure-related complication rates in PCNB. For this purpose, we studied a large single-center cohort of 235 consecutive patients with a range of thoracic benign and malignant lesions, and who underwent PCNB performed by a single experienced radiologist.
Patients and methodsPatientsBetween January 2013 and December 2014, 235 PCNB procedures were performed in 235 patients at our tertiary hospital. The population included 157 men (66.8%) and 78 women (33.2%) with a mean age of 58.48±18.0 years (median of 62.0 years) (Table 1). All biopsies were performed under CT guidance by one staff radiologist (DNC), who had, at the time of this study, more than 5 years’ experience in CT-TNB. Informed consent was obtained from all patients at least one day before the procedure. The study protocol was approved by the Institutional Ethics Committee.
Patient demographics and lesion characteristics.
| Patients | n | % | mean±s.d. (range) |
|---|---|---|---|
| Gender | |||
| Male | 157 | 66.8 | – |
| Female | 78 | 33.2 | – |
| Age, years: mean±s.d. | – | – | 58.48±18.0 (1-81) |
| Smoking | 116 | 49.4 | – |
| Emphysema | |||
| 0 | 113 | 48.1 | – |
| 1 | 83 | 35.3 | – |
| 2 | 36 | 15.3 | – |
| 3 | 3 | 1.3 | – |
| Lesion characteristics | n | % | mean±s.d. (range) |
|---|---|---|---|
| Localization | |||
| Mediastinum | 39 | 16.6 | – |
| Anterior | 18 | 7.6 | – |
| Middle | 10 | 4.3 | – |
| Posterior | 11 | 4.7 | – |
| Pulmonary | 196 | 83.4 | – |
| Right upper lobe | 75 | 31.9 | – |
| Middle lobe | 21 | 8.9 | – |
| Right lower lobe | 36 | 15.3 | – |
| Left upper lobe | 38 | 16.2 | – |
| Left lower lobe | 26 | 11.1 | – |
| Size (mm) | – | – | 22.13±7.16(9.0–47.0) |
| Pleural contact | 31 | 13.2 | – |
| Lesion depth from pleura (mm) | – | – | 17.73±7.81(1.0–39.0) |
| Contours | 10 | 4.3 | – |
| Spiculated/irregular | 218 | 92.8 | – |
| Regular | 17 | 7.2 | |
| Attenuation | |||
| Solid | 217 | 92.3 | – |
| Cystic | 2 | 0.8 | |
| Mixed (solid/cystic) | 16 | 6.8 | |
| Necrosis | 79 | 33.6 | – |
| Cavitations | 18 | 7.7 | – |
| Calcifications | 14 | 6.0 | – |
All examinations were strictly performed under clinical indications only. Patients were included if fulfilling one of the following criteria1: (a) suspected malignancy based on CT morphologic criterion, such as shape and contours; (b) a lesion initially regarded as benign/inflammatory but showing increase in size on follow-up or not responding to therapy/therapeutic test; (c) malignant lesion in which histological workup was expected to lead to a change in treatment strategy. Exclusion criteria encompassed: (a) lesions <5mm in maximum diameter; (b) lesions which showed strong enhancement on diagnostic CT and therefore were suspicious for being of vascular origin; (c) patients who were not able to follow verbal or visual instructions; (d) patients who refused to give their informed consent; and (e) history of bleeding tendency or alterations in blood parameters or coagulation profile.1,9
Biopsy protocol and techniquePatients receiving antiplatelet and/or anticoagulation agents discontinued treatment for 7 days prior to the biopsy. Before the procedure, the performing radiologist briefly reviewed the patient's history and previous CT scans, which were available for all patients before each biopsy. All biopsies were performed according to a standard protocol by the same radiologist (DNC) under CT guidance with a 16-row CT scanner (Brilliance 190P, Philips Medical Systems, Bothell, Washington, USA). After positioning the patient on the CT table in prone, supine or lateral decubitus (depending on lesion location), an initial pre-biopsy scan was obtained by acquiring the entire lesion on contiguous transverse sections. Sequentially, optimal access (skin entry site and needle path) was established on CT images; special care was taken in order to sample the lesion away from low-density areas suggestive of necrosis.5,9 After definition of the needle entry site, it was anaesthetized (2% lidocaine), the needle guide positioned and patients instructed to breathe calmly and to abstain from talking. The exposure parameters for CT scanning for pre-biopsy, biopsy and post-biopsy were: tube voltage 120kV, tube current 120 mAs and 2.5-mm collimation. Pre- and post-biopsy scans were reconstructed in 5-mm-thick transverse sections, overlap 5mm, lung window 2000/-500 HU and soft-tissue window 400/50 HU.
All biopsies were performed with a Manan Pro-Mag 2.2 non-coaxial biopsy system (Manan Scientific Products, Northbrook, IL, USA) always with an automatic 20-cm long cutting needle (Biocore II, Histo, Buenos Aires, Argentina); 20-mm-long tissue cores were retrieved. In cases in which gross naked eye inspection showed that an insufficient (less than 20-mm) tissue core was retrieved during the first biopsy, biopsy was repeated in the same session. The retrieved specimens were immediately immersed in 10% formalin solution and sent for histopathological analysis to the Department of Pathology of our hospital. The obtained material had value for immunohistochemical study, which was performed whenever necessary.
Immediate post-biopsy chest CT scans were performed to detect complications such as pneumothorax or hemorrhage. After the procedure, patients were placed on a stretcher or bed for a 4h-period, under the supervision of a nurse. Four hours after biopsy, new radiographs of the chest were obtained with the patient in erect position in order to detect delayed pneumothorax or hemorrhage. Pneumothorax was treated (after patients’ evaluation by a thoracic surgeon) in symptomatic or enlarging cases.1
Collected dataVariables that could potentially influence the diagnostic accuracy results and the frequency of complications were recorded. Age, sex, past or current smoking, and emphysema detected on CT slices were considered as patients’ characteristics. According to Montaudon et al. classification,19 emphysema was measured semi-quantitatively on the CT images performed before the biopsy (0, no emphysema; 1, some area of emphysema visible; 2, area of centrilobular emphysema or bullae distant from the needle tract; 3, prominent bullae or extensive emphysema close to the needle tract).
The second group of variables regards those related to the biopsied lesion, which included its size (in the two maximum orthogonal axial diameters as assessed on the pre-biopsy scan with adequate window and level settings),9 its location (anterior, middle, posterior mediastinum; intrapulmonary),1 segmentation (if intrapulmonary, classified as right upper lobe; middle lobe; right lower lobe; left upper lobe; left lower lobe), presence of a pleural contact, lesion depth from pleura (defined as the distance of the lesion from the pleural entry point of the needle), contours (spiculated/irregular or regular), attenuation (solid, cystic, or mixed), presence of necrosis, cavitations and calcifications.19 For the purposes of registering, pleural lesions originating from the lung were directly punctured and had pleural contact; intrapulmonary lesions were located in the lung parenchyma without pleural contact; mediastinal lesions were located in the mediastinum with or without contact to the mediastinal pleura.1
The third group of variables regards those related to the procedure, which included the position chosen for the biopsy (prone, supine or lateral decubitus), route of access to the lesion (anterior, posterior, lateral) and degree of difficulty (assessed prospectively and subjectively by the performer, ranging from 0, no difficulty, to 3, most difficult, also according to Montaudon et al. criteria19). In addition, when applicable (intrapulmonary and mediastinal lesions requiring pleural puncture), it was also recorded (a) the number of pleural passages20; (b) the “dwell time”, defined as the time between pleural puncture and needle removal21; and (c) the “needle-pleural angle, defined as the angle at which the needle enters the pleura.21 All distance and angle measurements were done with electronic calipers using the appropriate window settings.
Pneumothoraces at the time of the biopsy (as detected on CT), and after the biopsy (as detected on chest radiograph), were recorded, as well as the clinical outcome (chest tube drainage or conservative management). Hemoptysis and parenchymal hemorrhage visible on CT scans were also recorded, being classified as mild (haziness along needle track or in subsegmental air space), moderate (occurrence of less than five episodes of hemoptysis) and severe (hemoptysis or hemothorax associated with hemodynamic instability).1,16 Presence of mediastinal collections and need for in-hospital stay were also recorded. No deaths related to the procedure were identified during the realization of the study.
Definition of final diagnosis for the purposes of diagnostic accuracy analysesFor the purposes of conducting analyses of diagnostic accuracy in terms of malignancy, the lesions were initially classified as malignant or benign, with subsequent construction of 2×2 tables to correlate the result of the PCNB procedures with the final diagnosis, thereby finally calculating the levels of accuracy.
Final diagnoses were based on the surgical results, or on the clinical–radiological follow-up for a period of at least 12 months (response to first-line chemotherapy or antibiotic treatment designed on the basis of the biopsy; unchanged imaging findings in the absence of treatment).9 Considering malignancies as the main endpoint, the following possibilities were considered, based on the criteria previously defined by Priola et al.9 and Montaudon et al.19: (a) true positives for malignancy: those cases classified as malignant at PCNB and confirmed by histology of the surgical specimen; cytohistology of an additional biopsy from a distant metastatic lesion; imaging evidence (CT, MRI and/or PET-CT) of distant metastases; partial or complete response to first-line chemotherapy; (b) true negatives for malignancy (benign lesions), when a negative finding at PCNB had its benign nature confirmed by subsequent surgery or biopsy, or when the lesion appeared stable, smaller or had totally disappeared at 12-month follow-up CT in an untreated patient or in a patient treated with appropriate antibiotic (but not antineoplastic) therapy; (c) false positives for malignancy: all cases with a positive result on PCNB, but not confirmed by subsequent surgery; and (d) false negatives for malignancy: all cases with a negative or indeterminate result at PCNB (absence of cellular atypia, presence of nonspecific or granulomatous inflammatory reaction, insufficient material or blood contamination) with positive surgical outcome or disease progression at follow-up imaging.9,19
Statistical analysisThe sample characteristics are summarized in Table 1 as absolute and relative frequencies for categorical variables, and as means±standard deviations for continuous variables. Medians and ranges were also provided for asymmetric continuous variables. We also carried out diagnostic accuracy analyses (by correlating the results of the procedures with the final diagnosis), to obtain sensitivity, specificity, positive and negative predictive values, false positive and negative rates, and overall accuracy. Estimations of relative risk and logistic regression models were performed in order to assess possible associations between features (variables) related to patients, lesions and the procedure, and the main endpoints (malignancy and complications). The Student's t test was applied to continuous variable, with correction for unequal variances when required, and the Pearson's Chi-squared test (or the Fisher's Exact test, when applicable), for categorical variables. The threshold for statistical significance was set at p < 0.05. All analyses were performed in IBM SPSS Statistics, version 24 (IBM, Armonk, USA) software package.
ResultsPatients-, lesion- and procedure-related characteristicsTwo hundred and thirty-five consecutive patients fulfilled the inclusion criteria and were enrolled in this study. The patient and lesions characteristics are shown in Table 1. Most of patients were male (66.8%) and non-smokers (50.6%). Lesions were predominantly solid (217; 92.3%), located at the lungs (196; 83.4%), and with a right upper lobe predilection (75; 31.9%). Necrosis (79; 33.6%), cavitations (18; 7.7%) and calcifications (14; 6.0%) were less commonly found.
Procedure-related characteristics and complications are listed in Table 2. Most of procedures were performed with the patients positioned supine (172; 73.2%), through an anterior route (107; 45.5%), and with two or less pleural passages (187; 79.6%). Pneumothorax (49; 20.8%) and hemorrhage (37; 15.7%) occurred in a subset of patients, usually not requiring hospitalization (23; 9.8%).
Procedure-related characteristics and complications.
| Characteristic | n | % | mean±s.d. (range) |
|---|---|---|---|
| Position of patients | |||
| Prone | 55 | 23.4 | – |
| Supine | 172 | 73.2 | – |
| Lateral decubitus | 8 | 3.4 | |
| Route of access to the lesion | |||
| Anterior | 107 | 45.5 | |
| Posterior | 54 | 23.0 | |
| Lateral | 74 | 31.5 | |
| Degree of difficulty | |||
| 0 | 1 | 0.4 | – |
| 1 | 43 | 18.3 | – |
| 2 | 180 | 76.6 | – |
| 3 | 11 | 4.7 | – |
| Number of pleural passages | |||
| 0 | 4 | 1.7 | – |
| 1 | 4 | 1.7 | – |
| 2 | 179 | 76.2 | – |
| 3 | 38 | 16.2 | – |
| 4 | 10 | 4.2 | – |
| Dwell time (min) | – | – | 28.69±4.45(19.0–40.0) |
| Needle-pleural angle (°) | – | – | 77.77±6.84(60.0–87.0) |
| Pneumothorax | 49 | 20.8 | – |
| Conservative | 47 | 20.0 | – |
| Drainage | 2 | 0.8 | – |
| Hemorrhage | 37 | 15.7 | – |
| Mild | 35 | 14.9 | – |
| Moderate | 2 | 0.8 | – |
| Severe | 0 | 0 | – |
| Mediastinal collections | 12 | 5.1 | – |
| Hospitalization | 23 | 9.8 | – |
Specimen samples were successfully obtained from all the 235 cases. 155 lesions were finally considered malignant (Table 3), including 19 lesions after surgical resection and 136 lesions because they satisfied the established clinico-radiological criteria of follow-up. Among the 80 lesions finally considered to be benign, 10 lesions were confirmed benign after surgical resection and 70 were confirmed benign because they satisfied the established clinico-radiological criteria of follow-up. In total, there were 135 true-positive and 79 true-negative cases, as well as 1 false-positive and 20 false-negative cases (Table 4). Overall accuracy was 91.1%, with sensitivity of 87.1%, specificity of 98.8%, positive predictive value of 99.3%, and negative predictive value of 79.8%.
Final diagnoses in all patients submitted to CT-guided transthoracic biopsies confirmed through clinico-radiological follow-up or surgery.
| Diagnosis | Number of patients (%) |
|---|---|
| Malignant | 155 |
| Lung squamous cell carcinoma | 63 |
| Lung adenocarcinoma | 47 |
| Small cell lung cancer | 1 |
| Lung metastases | 19 |
| Mediastinal Non-Hodgkin lymphoma | 13 |
| Mediastinal Hodgkin lymphoma | 2 |
| Chondrosarcoma | 2 |
| Ganglioneuroblastoma | 1 |
| Mediastinal plasmacytoma | 2 |
| Lung carcinoid | 1 |
| Malignant thymoma | 4 |
| Benign | 80 |
| Nonspecific inflammation/pneumonia | 29 |
| Fungal infection | 17 |
| Lung tuberculosis | 12 |
| Cryptogenic organizing pneumonia | 5 |
| Fibrosis/sclerohyalinosis | 8 |
| Bronchogenic cyst | 2 |
| Thymic hyperplasia | 2 |
| Neurofibroma | 1 |
| Mediastinal leiomyoma | 1 |
| Goiter | 1 |
| Pulmonary chondroid hamartoma | 1 |
| Graft versus host-associated pulmonary disease | 1 |
Among the characteristics related to patients, age, presence of emphysema and smoking were associated with a positive diagnosis for malignancy after PCNB, but not sex. In terms of complications, sex was not associated with anyone, age was associated with increased risk for pneumothorax and slightly for hemorrhage, but not for mediastinal collections or hospitalization. Finally, emphysema and smoking were remarkably associated with increased risk for pneumothorax, hemorrhage and hospitalization, and have diminished the risk for presence of mediastinal collections (Table 5).
Patient-related characteristics versus final diagnosis of malignancy after PCNB and complication rates.
| Characteristic | Malignancy OR (95% CI); p value | ComplicationsOR (95% CI); p value | |||
|---|---|---|---|---|---|
| Pneumothorax | Hemorrhage | Mediastinal collections | Hospitalization | ||
| Emphysema | 3.19 (2.06–4.93); p<0.001 | 23.344 (9.897-55.064); p<0.001 | 1.671 (1.084-2.577); p=0.02 | 0.103 (0.014-0.743); p=0.02 | 2.999 (1.731-5.198); p<0.001 |
| Smoking | 3.23 (2.05–5.08); p<0.001 | 13.933 (5.277-36.788); p<0.001 | 2.450 (1.165-5.149); p=0.02 | 0.085 (0.011-0.672); p=0.02 | 2.560 (1.012–6.477); p<0.001 |
| Sex | 0.93 (0.77–1.12);p=0.445 | 0.763 (0.383–1.520); p=0.441 | 0.708 (0.324–1.549); p=0.387 | 2.097 (0.654–6.729); p=0.213 | 1.082 (0.438–2.673); p=0.865 |
| Age | 9.63 (4.89–14.36)p<0.001 | 1.102 (1.063-1.142); p<0.001 | 1.025 (1.001-1.050); p=0.039 | 0.975 (0.949-1.002); p=0.068 | 1.030 (0.999-1.062); p=0.058 |
Lesion features such as location, size, contours, presence of necrosis and calcifications were not significantly associated with malignancy (Table 6); solid attenuation, conversely, increased the risk for malignancy, as well as malignant lesions tended to be located deeper in relation to the pleura. Finally, presence of pleural contact, mixed attenuation and cavitations demonstrated to decrease the risk for a final diagnosis of malignancy (Table 6).
Lesion features and risk factors for malignancy.
| Characteristic | Malignancy (n or mean±SD) | OR (95% CI) | T test (t/F) | p value |
|---|---|---|---|---|
| Location | ||||
| Intrapulmonary | 125 | 1.89 (0.85–4.21) | 0.118 | |
| Anterior mediastinum | 15 | 0.36 (0.10–1.29) | 0.119 | |
| Middle mediastinum | 8 | 0.47 (0.09–2.27) | 0.349 | |
| Posterior mediastinum | 7 | 1.11 (0.31–3.92) | 0.868 | |
| Size | 2.16±0.71 | – | 1.48 (0.10) | 0.140 |
| Pleural contact | 5 | 0.07 (0.02–0.19) | <0.001 | |
| Lesion depth from pleura | 1.87±0.71 | – | −2.35/0.69 | 0.020 |
| Irregular or spiculated contours | 145 | 1.39 (0.51–3.80) | – | 0.521 |
| Regular contours | 10 | 0.72 (0.26–1.96) | 0.521 | |
| Attenuation | 1.03 (0.99–1.96) | 0.521 | ||
| Solid | 153 | 19.1 (4.27–85.6) | <0.001 | |
| Mixed | 2 | 0.06 (0.01–0.28) | <0.001 | |
| Cystic | 0 | – | – | |
| Necrosis | 52 | 1.01 (0.57–1.78) | – | 0.975 |
| Cavitations | 2 | 0.06 (0.01–0.28) | – | <0.001 |
| Calcifications | 9 | 0.93 (0.32–2.68) | – | 0.892 |
In terms of lesion features associated with complication rates (Table 7), intrapulmonary location was significantly associated with occurrence of pneumothorax and presence of mediastinal collections. Moreover, smaller lesions tended to be significantly associated with higher occurrence of pneumothorax and hemorrhage, whereas bigger lesions were associated with higher occurrence of mediastinal collections. Lesion size was not significantly associated with hospitalization rates. Absence of pleural contact was significantly associated with pneumothorax and hospitalizations.
Lesion features and technical factors versus occurrence of complications.
| Characteristic | Pneumothorax | Hemorrhage | Mediastinal collections | Hospitalization | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n or mean±SD) | t or χ2 | p value | (n or mean±SD) | t or χ2 | p value | (n or mean±SD) | t or χ2 | p value | (n or mean±SD) | t or χ2 | p value | |
| Location | ||||||||||||
| Intrapulmonary | 49 | 12.32 | 0.006 | 36 | 6.6 | 0.085 | 2 | 74.26 | <0.001 | 19 | 2.412 | 0.491 |
| Anterior mediastinum | 0 | 0 | 3 | 2 | ||||||||
| Middle mediastinum | 0 | 0 | 6 | 2 | ||||||||
| Posterior mediastinum | 0 | 1 | 1 | 0 | ||||||||
| Size | 1.84±0.66 | 4.206 | <0.001 | 1.92±0.54 | 2.731 | 0.006 | 2.80±0.92 | −2.694 | 0.003 | 2.12±0.88 | 0.641 | 0.522 |
| Pleural contact (yes/no) | (0/49) | 9.408 | <0.001 | (2/35) | 2.325 | 0.127 | (0/12) | 1.922 | 0.166 | (0/23) | 3.874 | 0.049 |
| Lesion depth from pleura | 2.73±0.58 | −16.28 | <0.001 | 1.67±0.70 | 1.05 | 0.294 | 1.80±0.40 | −0.016 | 0.987 | 2.52±0.70 | −4.773 | <0.001 |
| Contours (irregular and spiculated x regular) | (49/0) | 4.828 | 0.028 | (36/1) | 1.344 | 0.246 | (12/0) | 0.986 | 0.321 | (23/0) | 1.988 | 0.159 |
| Attenuation | ||||||||||||
| Solid | 49 | 5.135 | 0.077 | 36 | 1.578 | 0.454 | 11 | 0.151 | 0.927 | 22 | 0.474 | 0.789 |
| Mixed | 0 | 1 | 1 | 1 | ||||||||
| Cystic | 0 | 0 | 0 | 0 | ||||||||
| Necrosis (yes/no) | (19/30) | 0.738 | 0.390 | (9/28) | 1.699 | 0.192 | (5/7) | 0.367 | 0.545 | (13/10) | 5.994 | 0.020 |
| Cavitations (yes/no) | (21/18) | 5.135 | 0.020 | (3/34) | 0.012 | 0.911 | (0/12) | 1.049 | 0.306 | (0/23) | 2.115 | 0.146 |
| Calcifications (yes/no) | (1/38) | 1.695 | 0.193 | (2/35) | 0.024 | 0.877 | (1/11) | 0.127 | 0.721 | (1/22) | 0.118 | 0.731 |
| Position of patients | ||||||||||||
| Prone | 12 | 0.370 | 0.831 | 8 | 2.965 | 0.227 | 2 | 0.853 | 0.653 | 4 | 1.571 | 0.456 |
| Supine | 36 | 26 | 10 | 19 | ||||||||
| Lateral decubitus | 1 | 3 | 0 | 0 | ||||||||
| Route of access to the lesion | ||||||||||||
| Anterior | 19 | 12 | 3.111 | 0.211 | 8 | 10 | 0.128 | 0.959 | ||||
| Posterior | 14 | 1.490 | 0.475 | 10 | 2 | 2.342 | 0.310 | 5 | ||||
| Lateral | 16 | 15 | 2 | 8 | ||||||||
| Degree of difficulty | ||||||||||||
| 0-1 | 2 | 8.721 | 0.003 | 2 | 5.118 | 0.024 | 0 | 2.913 | 0.088 | 1 | 3.462 | 0.063 |
| 2-3 | 47 | 35 | 12 | 22 | ||||||||
| Number of pleural passages | 2.19±0.613 | 0.466 | 0.641 | 2.11±0.319 | 1.361 | 0.175 | 2.25±0.452 | −0.165 | 0.869 | 2.19±0.602 | 0.300 | 0.764 |
| Dwell time (min) | 28.3±4.10 | 0.640 | 0.523 | 28.4±3.94 | 0.370 | 0.712 | 29.75±3.86 | −0.852 | 0.395 | 28.45±4.459 | 0.250 | 0.803 |
| Needle-pleural angle (°) | 67.59±4.69 | 20.833 | <0.001 | 78.00±6.019 | −0.220 | 0.826 | 81.13±4.190 | −1.416 | 0.158 | 70.81±6.875 | 5.210 | <0.001 |
Deeper lesions in relation to the pleura were associated with higher rates of pneumothorax and hospitalizations, but not with hemorrhage or mediastinal collections. Irregular contours, on the other hand, were associated only with the occurrence of pneumothorax. Attenuation values and presence of calcifications were not associated with any of studied complications. Finally, necrosis in the lesion was associated with higher hospitalization rates, whereas presence of cavitations in the lesion was associated with higher indices of pneumothorax (Table 7).
Association between procedure-related technical factors versus complication ratesPosition of patients and the route of access to the lesion were not associated with any specific complication, as did not number of pleural passages and dwell time in minutes (Table 7). Conversely, higher degrees of difficulty (2 and 3 vs. 0 and 1) were significantly associated with higher pneumothorax and hemorrhage rates. Finally, smaller needle-pleural angles were associated with higher occurrence of pneumothorax and hospitalization, but not hemorrhage or mediastinal collections (Table 7).
DiscussionThe present study adds to the body of evidence in the radiological literature that supports the elevated diagnostic accuracy of PCNB in the process of confirming or excluding the malignant nature of a given thoracic lesion (irrespective of its mediastinal or lung origin) found in patients assisted within the context of everyday clinical practice. Furthermore, it also supports the concept that when performed by experienced hands this is a safe procedure, with low rates of complications and morbimortality. Finally, it dissects out the main variables inherent to patients, lesions and the procedure itself that are consistently associated with a final diagnosis of malignancy and/or occurrence of complications.
Diagnostic accuracy of PCNBWith the use of as a first and minimally invasive procedure for evaluating the nature of mediastinal and pulmonary lesions, this study obtained an overall diagnostic accuracy of 91.1%, with sensitivity of 87.1% and specificity of 98.8%. Although most studies focus preferably on lung lesions rather than on mediastinal ones, Priola et al.9 previously reported similar values of diagnostic accuracy for malignancy in a sample of 70 patients with 73 mediastinal lesions, reaching overall accuracy of 83.6%, with 83.6% sensitivity and 100% specificity.9 In terms of lung lesions, a recent meta-analysis encompassing 15 studies that analyzed the accuracy of CT-guided percutaneous core needle biopsy showed sensitivity values ranging from 84% to 97% (pooled 95%, 95% CI 0.93-0.96), and specificity ranging from 91% to 100% (pooled 99%, 95% CI: 0.98-1.00). The values found in the present study, therefore, absolutely fit and support those previously reported elsewhere.
Complication rates of PCNBPneumothorax (20.8%) and hemorrhage (15.7%) were the two most complications related to the procedure, with the need for hospitalization occurring in only a subset of these patients (9.8%); mediastinal collections were rare (5.1%). When considering only the universe of patients with mediastinal lesions, Priola et al.9 reported lower values, with an overall complication rate of 6.8% (5/73 procedures), which in most cases (4/5 complications, 80%) were of no clinical relevance, requiring no treatment or hospitalization; pneumothorax was the most frequent complication, occurring in 5.5% patients (4/73 procedures). Other authors addressing mediastinal lesions, however, have been reporting rates falling within a range of 4.6% to 42.7% of pneumothorax, for example.12,22–25 Relative to lung lesions, a recent meta-analysis26 demonstrated that, for PCNB (32 articles; 8133 procedures) pooled overall complication rate was 38.8% (95% CI: 34.3–43.5%), being 25.3% for pneumothorax and 18.0% for hemorrhage; remarkably, overall complication rate was higher for PCNB compared to PNAB.26 Major complications, such as pneumothorax requiring intervention, hemorrhage, and death were low (5.7%)26; in our study there were only two cases of pneumothorax requiring drainage (0.8%) and two cases of moderate hemorrhage (0.8%). We also highlight the occurrence of two cases of mediastinal collections complicating biopsy of intrapulmonary lesions; both occurred in elderly patients, in whom a paramediastinal approach to anterior segments was performed by transfixing the anterior mediastinum owing to the impossibility of direct access (small and deep lesions in patients with severe sternoclavicular joint hypertrophy). Once again, our reported values for major and minor complications fall within the range described in the literature.
Patient-related characteristics, malignancy and complication ratesAs expected, the classical, largely and well-known association between lung emphysema, smoking and malignancy was also present in our sample. Chronic obstructive pulmonary disease (COPD) is one of the most well established independent risk factors for the development of lung cancer, and tobacco use was long believed to be the only link between the two diseases.27 Emphysema and smoking in our sample were also found to be remarkably linked to increased risk for pneumothorax, hemorrhage and hospitalization. The question whether emphysema per se is or not a risk factor for the development of pneumothorax after CT-TNB remains a controversial issue. A recent review reported that a combination of distinct variables such as lesion size, lesion depth and the presence of emphysema concurs to determine the incidence of iatrogenic pneumothoraces.28 Additionally, while some studies showed no influence of emphysema on the incidence of pneumothoraces,29 others demonstrate that the presence of emphysema as quantified by CT volumetric lung analysis is an independent predictor of pneumothorax after CT-TNB.30 Results of the present study add evidence favoring the presence of this association, also in accordance with other novel publications.31 Conclusions about the positive association between hemorrhage and emphysema are less evident in the literature. Older age, another variable positively related to occurrence of complications in our study, is another also traditionally linked to higher incidence of procedure-related complications.26
Lesion features, malignancy and complication ratesThe expected absence of significant association between malignancy and most of the features related to the lesions (such as location, size, contours, presence of necrosis and calcifications) highlights the notion that although such features may help to assess to some extent the likelihood of malignancy in the clinical practice, a definitive diagnosis cannot be achieved only on the basis of the image, as most of these features are unspecific and common to both malignant and non-malignant lesions.32 The particular inverse association between presence of pleural contact, mixed attenuation and cavitations seems to be fortuitous and only a peculiarity related to our sample of patients, as these characteristics hitherto have not proven useful in distinguishing between malignant and benign lesions. For example, in our sample just 5 patients with lesions showing pleural contact had a final diagnosis of malignancy (against 26 who had lesions with pleural contact and a final diagnosis of benignity); however, such an unspecific feature is known to occur in a range of conditions as wide as from pneumonias to adenocarcinomas.33 The same also applies to cavitations, which in our sample occurred mostly in patients with fungal and TB lesions, although such a feature may also be found in a range of malignant neoplasms.34
The association between lesion features and complications, on the other side, is more straightforward. Deeper lesions (as lung lesions tend to be as compared to mediastinal lesions, which can be usually assessed directly through an anterior or posterior route) and lesion size (factors significantly linked to complications in our sample) have traditionally been appointed as associated to higher rates of complications in numerous studies and meta-analyses.1,26,35,36
Procedure-related technical factors and complication ratesIn our study, position of patients, route of access to the lesion, number of pleural passages and dwell time were not associated to occurrence of complications. This is in accordance with large and important studies that failed previously to demonstrate a significant association between such variables.21,37 Topal et al., 37 for example, in a study encompassing 453 procedures, demonstrated that depth of the lesion (p<0.001) and severity of the emphysema (p<0.01) were associated to pneumothorax, but not the number of passages and the level of experience of the operator. Ko et al., 21 studying 159 patients, found that longer dwell times do not correlate with pneumothorax and should not influence the decision to obtain more biopsy samples.21 These authors, however, also demonstrated that smaller needle-pleural angles (<80 degrees in their sample) significantly correlated with higher occurrence of pneumothorax,21 a finding also observed in our study, in which a mean of about 68° for the angle was found in the group with pneumothorax, against 81° in the group without pneumothorax. It is important to emphasize, however, that the recent meta-analytic approach to the question of procedure-related variables did not demonstrate any significant association with occurrence of complications when analyzing specifically the subgroup of PCNB.26
Strengths and limitationsOur study has the advantages of a) addressing homogeneously the question of usage of PCNB rather than mixing the results with those obtained through utilization of PNAB; b) employing a large sample of consecutively selected subjects, avoiding bias selection; c) reflecting a real life scenario of the standard clinical practice, in which both mediastinal and lung lesions (and not just one subtype to the detriment of another) must be addressed; d) avoiding the bias introduced by the employment of a heterogeneous range of performers when computing the overall accuracy of a procedure; e) the solid criteria adopted for clinical and radiological follow-up in order to determine the malignant nature of a lesion. Moreover, particularly when studying the occurrence of complications, most studies report patient-, lesion- and procedure-related features only as a mean, and complications are not stratified based on these variables,26 a common limitation that we have specifically surpassed here.
We acknowledge, however, that our study is not without limitations. First, its retrospective observational nature, which prevented us from a) investigating the rationale behind the choice of PCNB instead of PNAB for each procedure, a factor that, as previously demonstrated, has influence on the occurrence of complications26; b) establishing two arms (PNAB versus PCNB) designed to be prospectively assessed. Second, the design of our study, in which all procedures were performed by only one operator, although allowing us to confidently establishing overall diagnostic accuracy rates, in contradistinction also prevented us of assessing the effect of operator experience in the occurrence of complications, a factor that intuitively is thought to influence such rates26. However, unlike most studies, we assessed the subjective degree of the procedure by the operator according to the criteria by Montaudon et al.19 and found a significant association between the occurrence of complications such as pneumothorax and hemorrhage and the degree of difficulty, which, to our eyes, is an alternative and appropriate method of assessing this question in such a context. Third, regarding procedure-related hemorrhagic complications, sharing a limitation with some previous studies, we also have not assessed the presence of pulmonary hypertension, which could have be inferred from previous CT studies by measuring the diameter of the pulmonary arteries and veins.
Fourth, subsolid lung nodules (ground-glass and part-solid nodules), which represents a growing and significant number of lung nodules that are now being described in many CT studies,38 were not assessed in the present study given the protocols previously in force at our institution at the time of its design, as well as of the previously limited experience with biopsy of such nodules. However, as we have obtaining increasing experience and performing a growing number of procedures in subsolid lesions, the results of a new study focusing only them are expected to be reported soon. Finally, owing to the lack of institutional availability at the time of this study, we had not available supplementary data provided by PET/CT for predicting the malignant or benign nature of a thoracic lesion when reviewing the patients’ history and imaging studies. PET/CT may not only shed light regarding the nature of a lesion, but may also detect unsuspected metabolically active lesions within the mediastinum or supraclavicular areas that could have changed the diagnostic approach in some cases.39 It should be pointed out, on the other hand, that some recent studies found that having undergone PET/CT prior to biopsy did not influence the diagnostic accuracy in patients with lung lesions and that sampling should not be delayed based on PET/CT availability.40
ConclusionPCNB is a safe procedure with high diagnostic accuracy for the clinical work up of patients with indeterminate mediastinal and lung lesions requiring a histological diagnosis. Pneumothorax and hemorrhage are the most common complications. Emphysema, smoking, older age, depth and smaller size of lesions, and smaller needle-pleural angles are the most relevant factors related to the occurrence of complications.
Conflict of interestThe authors declare no conflict of interests