Idiopathic pulmonary fibrosis (IPF) is an irreversible and progressive fatal interstitial lung disease with a poor prognosis. The aim of this study is to investigate the predictive value of combined blood biomarkers, pulmonary function and quantitative monitoring by computer-aided diagnosis (CAD) system in IPF patients.
MethodsPulmonary baseline function and pathological features of 126 patients with IPF were analyzed using spirometry and chest X-ray. Patients were divided into survival group and non-survival group after 5 years follow-up. The relationships the levels of peripheral blood biomarkers, quantitative imaging characteristics and pulmonary function were analyzed between the two groups.
ResultsThe baseline level of serum Krebs von den Lungen-6 (KL-6) and C-X-C motif chemokine 13 (CXCL13) were moderately or highly correlated with annual changes in forced vital capacity (FVC), carbon monoxide diffusing capacity (DLCO), total lung capacity (TLC), total interstitial lung disease (ILD) lesions, and the volume changes of reticular. The baseline level of serum KL-6 was higher than the cut-off value of 800.0U/ml and baseline level of serum CXCL13 was higher than the cut-off value of 62.0pg/ml. IPF patients with baseline levels of serum KL-6 and CXCL13 lower than the cut-off value had longer median survival time.
ConclusionsSerum KL-6 and CXCL13 may be predictive biomarkers for the outcomes of patients with IPF patients and their baseline levels were related to the progression of pulmonary function and quantitative monitoring by CAD system.
La fibrosis pulmonar idiopática (FPI) es una enfermedad intersticial pulmonar irreversible, progresiva y mortal con mal pronóstico. El objetivo de este estudio es investigar el valor predictivo combinado de los marcadores en sangre, la función pulmonar y el seguimiento cuantitativo mediante sistemas de diagnóstico asistido por ordenador (DAO) en pacientes con FPI.
MétodosSe analizaron la función inicial pulmonar y los criterios patológicos de 126pacientes con FPI mediante espirometría y radiografía de tórax. Los pacientes se dividieron en grupo de supervivencia y grupo de no supervivencia a los 5años de seguimiento. Se comparó la relación entre los niveles de marcadores en sangre periférica, las características cuantitativas en la prueba de imagen y la función pulmonar entre ambos grupos.
ResultadosLos niveles iniciales de Krebs von den Lungen-6 (KL-6) y la quimiocina de motivo CXC 13 (CXCL13) en suero se correlacionaron moderada o fuertemente con los cambios anuales en la capacidad vital forzada (FVC), la capacidad de difusión de monóxido de carbono (DLCO), la capacidad pulmonar total (TLC), el total de las lesiones de enfermedad pulmonar intersticial (EPI) y cambios en el volumen de densidades reticulares. El nivel basal de KL-6 en suero fue mayor que el valor de corte de 800U/ml y el nivel basal de CXCL13 en suero fue mayor que el valor de corte de 62pg/ml. Los pacientes con FPI y niveles iniciales en suero de KL-6 y CXCL13 más bajos que el valor de punto de corte presentaron una mediana mayor de tiempo de supervivencia.
ConclusionesLos valores en suero de KL-6 y CXCL13 podrían servir como biomarcadores predictivos para el pronóstico de los pacientes con FPI. Sus niveles al inicio del estudio se relacionaron con la progresión de la función pulmonar y el seguimiento cuantitativo con el sistema DAO.
Idiopathic pulmonary fibrosis (IPF), which has the histological pattern of usual interstitial pneumonia (UIP), is an irreversible and progressive fatal interstitial lung disease with a poor prognosis.1 Unfortunately, there is no effective drug for the treatment of IPF, and the median survival is about 3–5 years following diagnosis. The developmental trajectories of IPF vary greatly and can be divided into three categories: stable or slow progress; Rapid deterioration; Steady but interspersed with periods of rapid progress.2 Currently, there is no widely accepted IPF disease monitoring system, so it is difficult for clinicians to predict the disease process of each patient. Therefore, the search for new biomarkers from the underlying mechanism may provide a new method for the prognosis evaluation of IPF.
Krebs von den Lungen-6 (KL-6) is a high-molecular-weight glycoprotein expressed on the extracellular surface of alveolar type II cells and bronchial epithelial cells.3 As a chemokine, it can promote migration, proliferation and survival of pulmonary fibroblasts.4 In interstitial lung diseases, KL-6 has been studied as a nonspecific marker of fibrosis. Previous study found that the serum KL-6 level of IPF patients was significantly increased compared with healthy volunteers or other patients with interstitial lung diseases.5 C-X-C motif chemokine 13 (CXCL13) is a key mediator that guides B cells to inflammatory lesions, which is significantly elevated in many autoimmune syndromes and is associated with clinical activity in these diseases.6,7 C-X-C chemokine receptor 5 (CXCR5) is the B cell ligand of CXCL13, which has the role of mediating chemotaxis of lymphocytes.8 Repeated antigen stimulation enhances B-cell differentiation, another typical characteristic of systemic lupus erythematosus, rheumatoid arthritis, and many other autoantibody syndromes, which are also clinically relevant to IPF.9 Abnormal B cells were recently found in the pathogenesis of IPF, suggesting that CXCL13 may be a biomarker related to IPF etiology and reflecting the progress of fibrosis. Previous studies have shown that serum KL-6 and CXCL13 can be used as biomarkers for the diagnosis of IPF and the judgment of prognosis. However, its sensitivity and specificity to the prognosis of IPF are still unclear, and most of them are retrospective small sample size studies, the clinical application value still needs to be further discussed.
In this study, we aimed to identify the relationship between serum KL-6 and CXCL13 levels, computer-aided diagnosis (CAD) system for automatic quantitative analysis and objective indicators of pulmonary function, and determined the role of complex indicators including KL-6 and CXCL13 levels, pulmonary function, and CAD system for automatic quantitative analysis as markers on reasonable prediction of disease progression and death risk of IPF.
MethodsParticipantsIn this study, we enrolled participants (n=126) who diagnosed and followed up in departments of Sichuan Provincial People's Hospital from January 1, 2012 to December 31, 2017 and met the diagnostic criteria for IPF. All participants gave written informed consent and the study was approved by the Ethics Review Board at The University of Electronic Science and Technology of China. The associated permit numbers is 2017-171. All of the patients had given their informed consent.
IPF Diagnostic and Exclusion CriteriaIPF diagnosis was based on the American Thoracic Society and European Respiratory Society guidelines.10 The diagnostic criteria were as follows: (i) other known causes of interstitial lung disease in the subjects were excluded; (ii) patients presented typical UIP on high resolution computed tomography (HRCT)11; and (iii) patients with atypical HRCT received underwent surgical lung biopsy, and HRCT and lung biopsy pathology were in accordance with the particular combination of UIP. The exclusion criteria were as follows: (i) patients had other types of high resolution computed tomography interstitial lung disease (ILD); (ii) IPF patients with lung transplantation; (iii) IPF patients who lost follow-up; and (iv) IPF patients with malignant tumors.
Study DesignWe performed a single-center prospective cohort study to evaluate the rationality of using compound indicators such as serum levels of KL-6 and CXCL13, pulmonary function and quantitative analysis of CAD system to predict the progress and mortality risk of IPF.
The follow-up plan included the first visit (time-point 0), follow-up period and follow-up end point. In this study, we reviewed the clinical indicators every 3 months, and the chest HRCT every 6 months in the first year (a total of 2 times). After the first year, HRCT examinations were performed as required.
HRCT EvaluationThe HRCT investigation was performed using a SOMATOM Definition Flash (Siemens, Germany). The results were evaluated by two experienced consultant and CAD system for automatic quantitative analysis, respectively. The rating system for visual assessment consists of four imaging anomalies within a particular region12: pulmonary emphysema, ground-glass opacity (GGO), grid shadow, and honeycombing (HC) lesions. According to the proportion of pulmonary emphysema, GGO, reticular lesions, HC lesions, and overall ILD lesions (including GGO, reticular lesions, and HC lesions) in the total lung mass, each score is 0–5 points, 0 points for those without lesions, accumulation range <5% for 1 point, 5%–25% for 2 points, 25%–50% for 3 points, 50%–75% for 4 points and 75% for 5 points.
Enzyme-linked Immunosorbent Assay (ELISA) and Cytokine ArrayA Human ELISA Kit (KL-6) was obtained from FUJIREBIO INC. CXCL13 (C-X-C Motif chemokine 13) BioAssay ELISA Kit (Human) was obtained from Cayman Chemical Company. Cytokines were detected according to the manufacturer's instructions. Absorbance was measured at 450nm on a microplate reader (Thermo Fisher Scientific, Inc.).
Statistical AnalysisStatistical analysis was performed using SPSS20.0 software (IBM Corp., Armonk, NY, USA). The normal distribution measurement data were presented as the means±standard deviation, and differences among multiple groups were compared by one-way analysis of variance. The measurement data of skew distribution were analyzed by non-parametric method. The count data were represented as the rate and constituent ratio, and Pearson's χ2 test was applied for analysis. The linear regression was applied for analysis of relationships between serum KL-6 and CXCL13 levels, pulmonary function and CAD system for automatic quantitative analysis. The ROC curves were applied to compare the sensitivity and specificity of serum markers and other indicators in the prognosis diagnosis of IPF patients. Multivariate regression analysis was performed including parameters that were significantly associated with mortality, invariably, parameters that are considered obligatory (age and sex). The cut-off value of serum markers was determined using the Yoden index. The survival function was estimated by Kaplan–Meier method and compared by log-rank test.
ResultsAnalysis of Baseline Clinical Characteristics in PatientsThere were 126 patients who met the entry criteria for this study. Among them, 11 cases (8.7%) were confirmed as UIP pathology by surgical lung biopsy or post-lung transplantation biopsy. The remaining 115 cases (91.3%) were diagnosed as typical UIP mode by HRCT. Baseline age in the non-survival group was significantly higher than those in the survival group (P<.05). Baseline gender and ex-smoker did not differ significantly between survival group and non-survival group (P>.05) (Table S1).
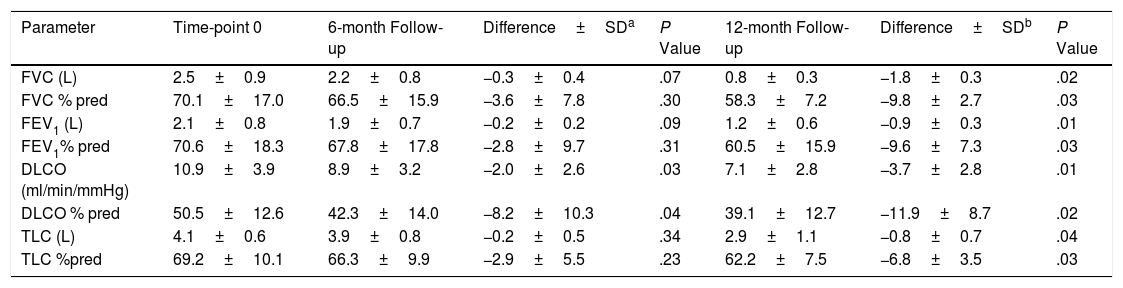
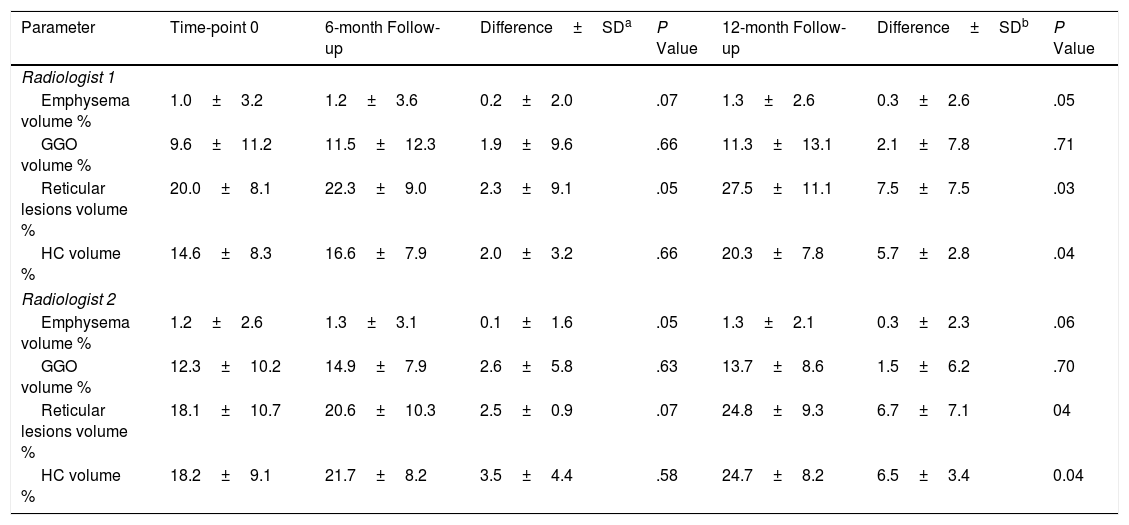
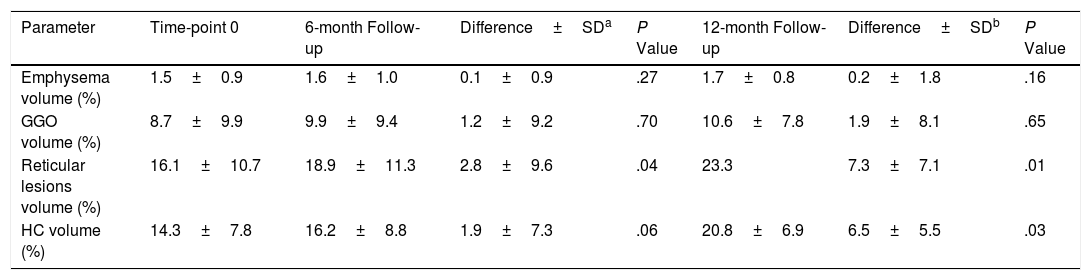
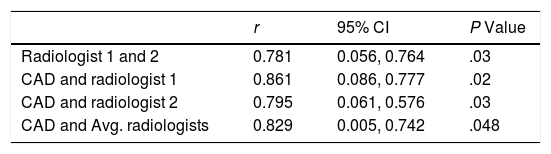
The Changes of Pulmonary Function and Lung Lesions of IPF Patients During a 12-month Follow-up PeriodBefore a week of HRCT scans, we measured the pulmonary function accordance with standard guidelines.13 As shown in Table 1, it revealed that pulmonary function including forced vital capacity (FVC), forced expiratory volume in one second (FEV1), carbon monoxide diffusing capacity (DLCO) and total lung capacity (TLC) were significantly decreased during a 12-month follow-up period compared with time-point 0 (P<.05). Then, the lung volume differences of lung lesions were evaluated by two radiologists and CAD system. As shown in Tables 2 and 3, the lung lesions of IPF patients including reticular lesions volume and HC volume were significantly increased during a 12-month follow-up period compared with time-point 0 (P<.05). As shown in Table 4, the correlation between the visual semi-quantitative score of disease severity between the two radiologists was good (r=0.781). The correlation between CAD system and radiologist visual semi-quantitative scoring system also was good, and the correlation coefficients were 0.861(Radiologist 1) and 0.795 (Radiologist 2), respectively. The correlation between CAD system and the average score of the two radiologists was 0.829.
Differences of Pulmonary Function in the Study Population During 12-month Period.
| Parameter | Time-point 0 | 6-month Follow-up | Difference±SDa | P Value | 12-month Follow-up | Difference±SDb | P Value |
|---|---|---|---|---|---|---|---|
| FVC (L) | 2.5±0.9 | 2.2±0.8 | −0.3±0.4 | .07 | 0.8±0.3 | −1.8±0.3 | .02 |
| FVC % pred | 70.1±17.0 | 66.5±15.9 | −3.6±7.8 | .30 | 58.3±7.2 | −9.8±2.7 | .03 |
| FEV1 (L) | 2.1±0.8 | 1.9±0.7 | −0.2±0.2 | .09 | 1.2±0.6 | −0.9±0.3 | .01 |
| FEV1% pred | 70.6±18.3 | 67.8±17.8 | −2.8±9.7 | .31 | 60.5±15.9 | −9.6±7.3 | .03 |
| DLCO (ml/min/mmHg) | 10.9±3.9 | 8.9±3.2 | −2.0±2.6 | .03 | 7.1±2.8 | −3.7±2.8 | .01 |
| DLCO % pred | 50.5±12.6 | 42.3±14.0 | −8.2±10.3 | .04 | 39.1±12.7 | −11.9±8.7 | .02 |
| TLC (L) | 4.1±0.6 | 3.9±0.8 | −0.2±0.5 | .34 | 2.9±1.1 | −0.8±0.7 | .04 |
| TLC %pred | 69.2±10.1 | 66.3±9.9 | −2.9±5.5 | .23 | 62.2±7.5 | −6.8±3.5 | .03 |
Lung Volume Differences of Lung Lesions Evaluated by Radiologists During 12-month Period.
| Parameter | Time-point 0 | 6-month Follow-up | Difference±SDa | P Value | 12-month Follow-up | Difference±SDb | P Value |
|---|---|---|---|---|---|---|---|
| Radiologist 1 | |||||||
| Emphysema volume % | 1.0±3.2 | 1.2±3.6 | 0.2±2.0 | .07 | 1.3±2.6 | 0.3±2.6 | .05 |
| GGO volume % | 9.6±11.2 | 11.5±12.3 | 1.9±9.6 | .66 | 11.3±13.1 | 2.1±7.8 | .71 |
| Reticular lesions volume % | 20.0±8.1 | 22.3±9.0 | 2.3±9.1 | .05 | 27.5±11.1 | 7.5±7.5 | .03 |
| HC volume % | 14.6±8.3 | 16.6±7.9 | 2.0±3.2 | .66 | 20.3±7.8 | 5.7±2.8 | .04 |
| Radiologist 2 | |||||||
| Emphysema volume % | 1.2±2.6 | 1.3±3.1 | 0.1±1.6 | .05 | 1.3±2.1 | 0.3±2.3 | .06 |
| GGO volume % | 12.3±10.2 | 14.9±7.9 | 2.6±5.8 | .63 | 13.7±8.6 | 1.5±6.2 | .70 |
| Reticular lesions volume % | 18.1±10.7 | 20.6±10.3 | 2.5±0.9 | .07 | 24.8±9.3 | 6.7±7.1 | 04 |
| HC volume % | 18.2±9.1 | 21.7±8.2 | 3.5±4.4 | .58 | 24.7±8.2 | 6.5±3.4 | 0.04 |
Lung Volume Differences of Pulmonary Lesions Detected by CAD System During 12-month Period.
| Parameter | Time-point 0 | 6-month Follow-up | Difference±SDa | P Value | 12-month Follow-up | Difference±SDb | P Value |
|---|---|---|---|---|---|---|---|
| Emphysema volume (%) | 1.5±0.9 | 1.6±1.0 | 0.1±0.9 | .27 | 1.7±0.8 | 0.2±1.8 | .16 |
| GGO volume (%) | 8.7±9.9 | 9.9±9.4 | 1.2±9.2 | .70 | 10.6±7.8 | 1.9±8.1 | .65 |
| Reticular lesions volume (%) | 16.1±10.7 | 18.9±11.3 | 2.8±9.6 | .04 | 23.3 | 7.3±7.1 | .01 |
| HC volume (%) | 14.3±7.8 | 16.2±8.8 | 1.9±7.3 | .06 | 20.8±6.9 | 6.5±5.5 | .03 |
Correlation Between the Scores From Radiologists and CAD Results.
| r | 95% CI | P Value | |
|---|---|---|---|
| Radiologist 1 and 2 | 0.781 | 0.056, 0.764 | .03 |
| CAD and radiologist 1 | 0.861 | 0.086, 0.777 | .02 |
| CAD and radiologist 2 | 0.795 | 0.061, 0.576 | .03 |
| CAD and Avg. radiologists | 0.829 | 0.005, 0.742 | .048 |
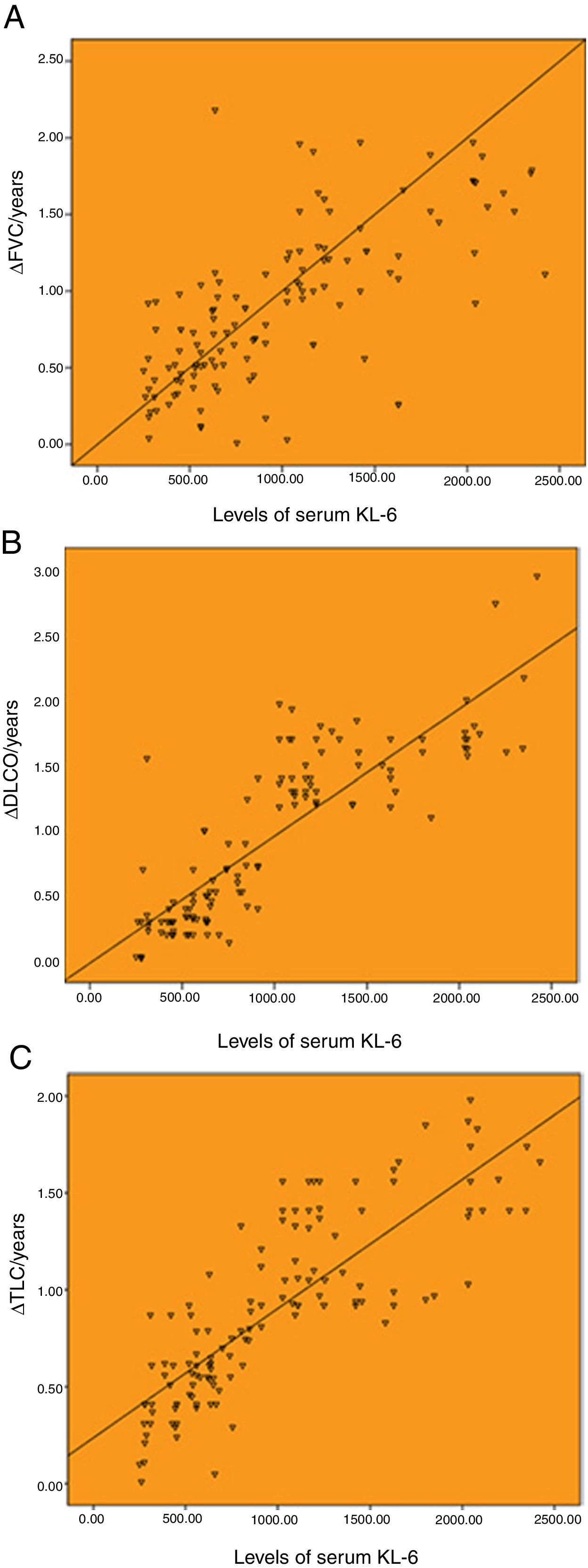
Correlation between the baseline levels of serum KL-6 and annual change of pulmonary function.
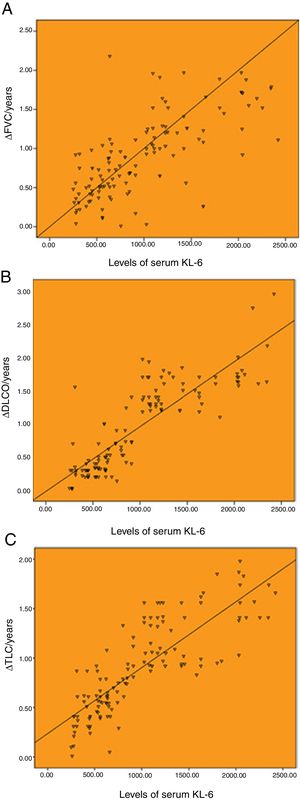
As shown in Fig. 1, the baseline levels of serum KL-6 was highly correlated with annual changes of pulmonary function. The serum KL-6 level was highly correlated with ΔFVC/years, ΔDLCO/years and ΔTLC/years, with the correlation coefficients of r=0.707, r=0.859 and r=0.827 (P<.001), respectively.
Correlation between the baseline levels of serum KL-6 and annual change of pulmonary function. (A) Correlation between the baseline levels of serum KL-6 and annual change of FVC (r=0.707). (B) Correlation between the baseline levels of serum KL-6 and annual change of DLCO (r=0.859). (C) Correlation between the baseline levels of serum KL-6 and annual change of TLC (r=0.827). ΔFVC/year, ΔDLCO/year and ΔTLC/year represent the annual change value of FVC, DLCO and TLC, respectively.
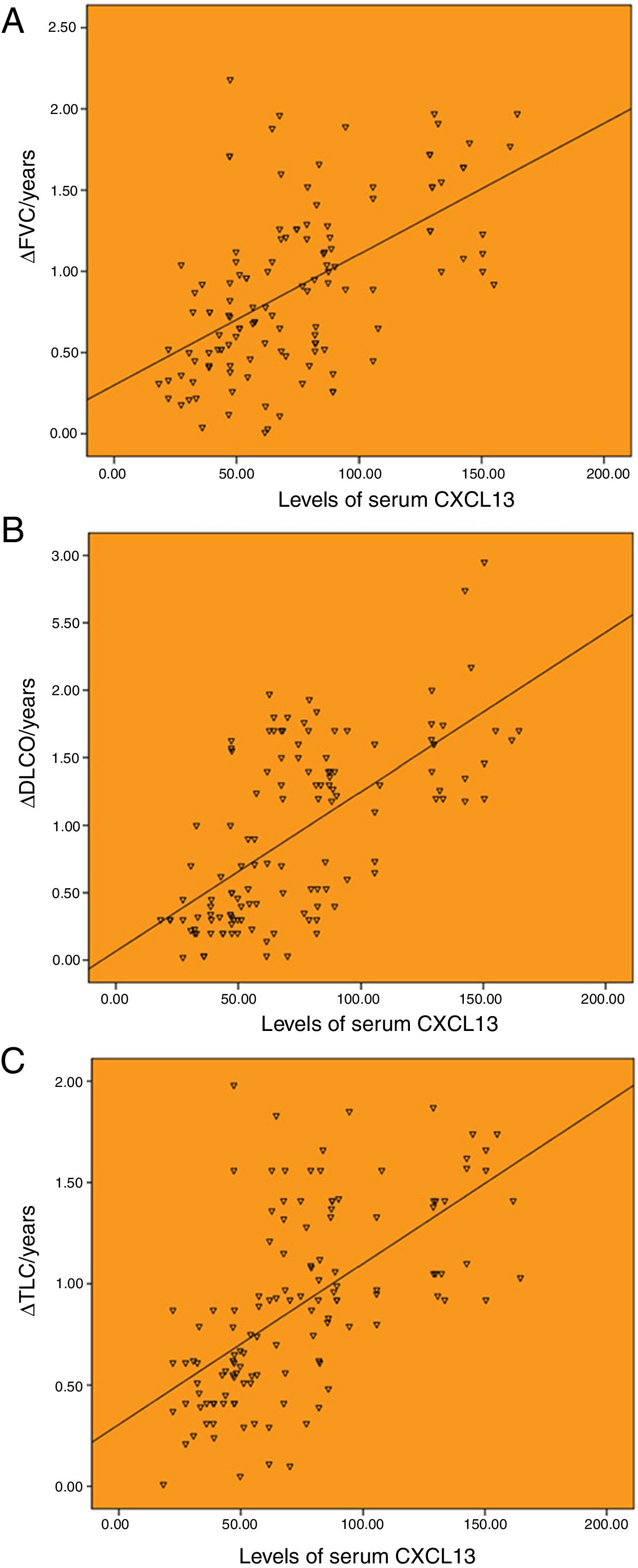
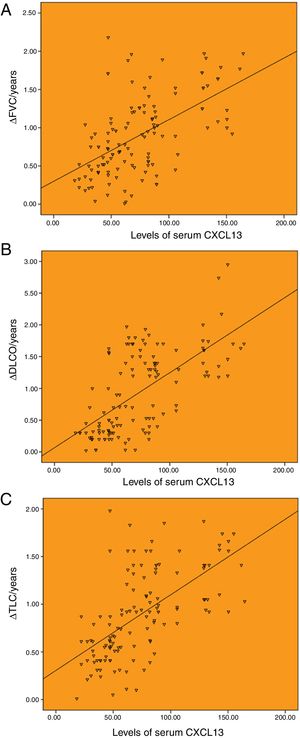
There was a significant correlation between serum CXCL13 baseline levels and annual changes of pulmonary function. Among them, serum CXCL13 level was moderately correlated with ΔFVC/years, with a correlation coefficient of r=0.561 (P<.001; Fig. 2A). The serum CXCL13 level was highly correlated with ΔDLCO/year and ΔTLC/year, with the correlation coefficients of r=0.648 and r=0.612 (P<.001), respectively (Fig. 2B and C).
Correlation between the baseline levels of serum CXCL13 and annual change of pulmonary function. (A) Correlation between the baseline levels of serum CXCL13 and annual change of FVC (r=0.561). (B) Correlation between the baseline levels of serum CXCL13 and annual change of DLCO (r=0.648). (C) Correlation between the baseline levels of serum CXCL13 and annual change of TLC (r=0.612).
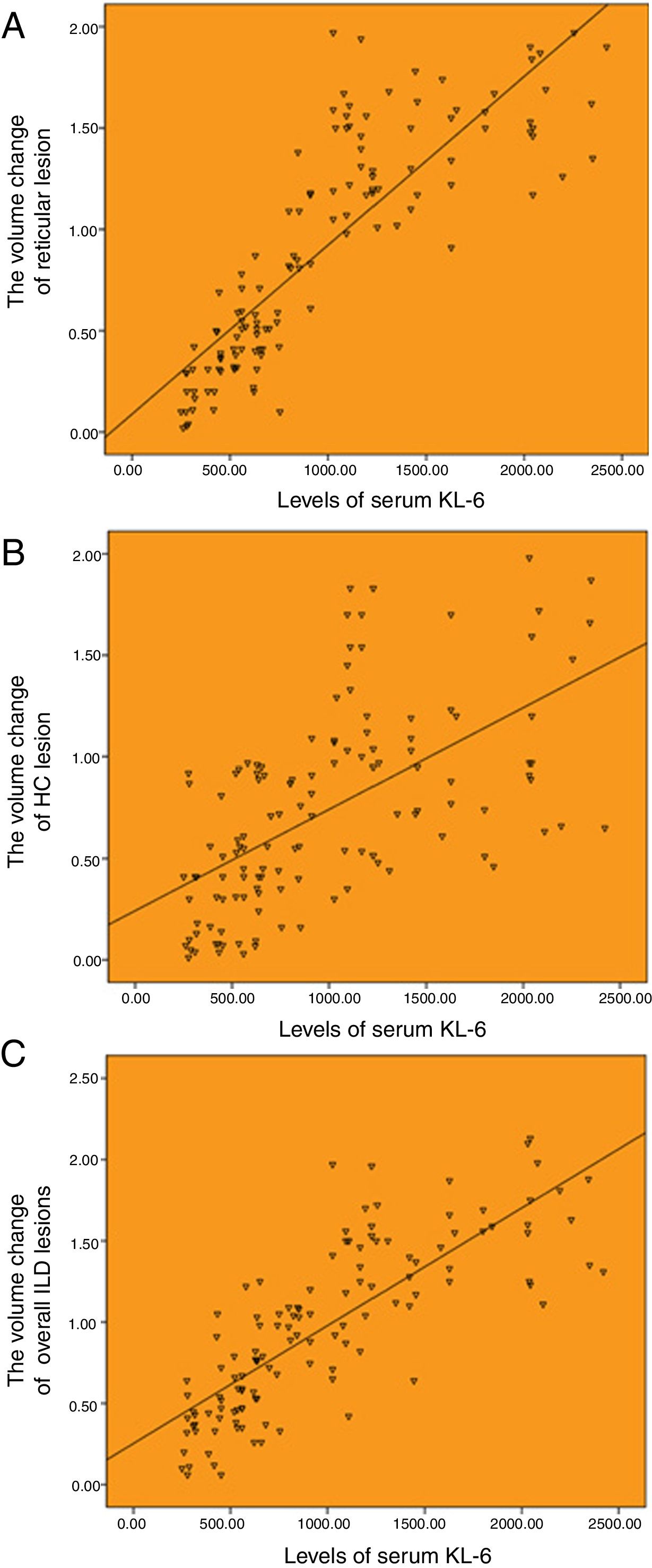
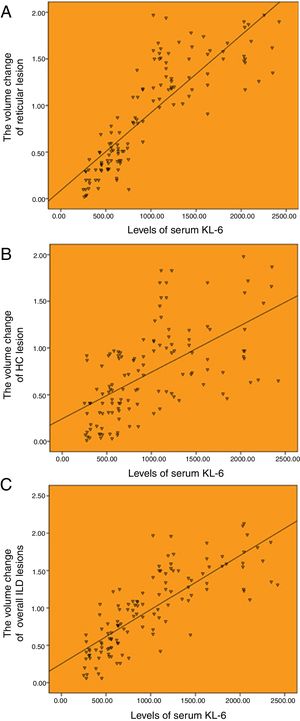
There was a good correlation between serum KL-6 baseline level and HRCT progression. The serum level of KL-6 was highly correlated with the annual increased volume of reticulation, and the annual increased volume of overall ILD lesions, with the correlation coefficients of r=0.850 and r=0.803 (P<.001), respectively (Fig. 3A and C). And moderately correlated with the annual increased volume of honeycombing, with a correlation coefficients of r=0.504 (P<.001; Fig. 3B).
Correlation between the baseline levels of serum KL-6 and HRCT progression. (A) Correlation between the baseline levels of serum KL-6 and annual increased volume of reticulation (r=0.850). (B) Correlation between the baseline levels of serum KL-6 and annual increased volume of honeycombing (r=0.504). (C) Correlation between the baseline levels of serum KL-6 and annual increased volume of overall ILD lesions (r=0.803).
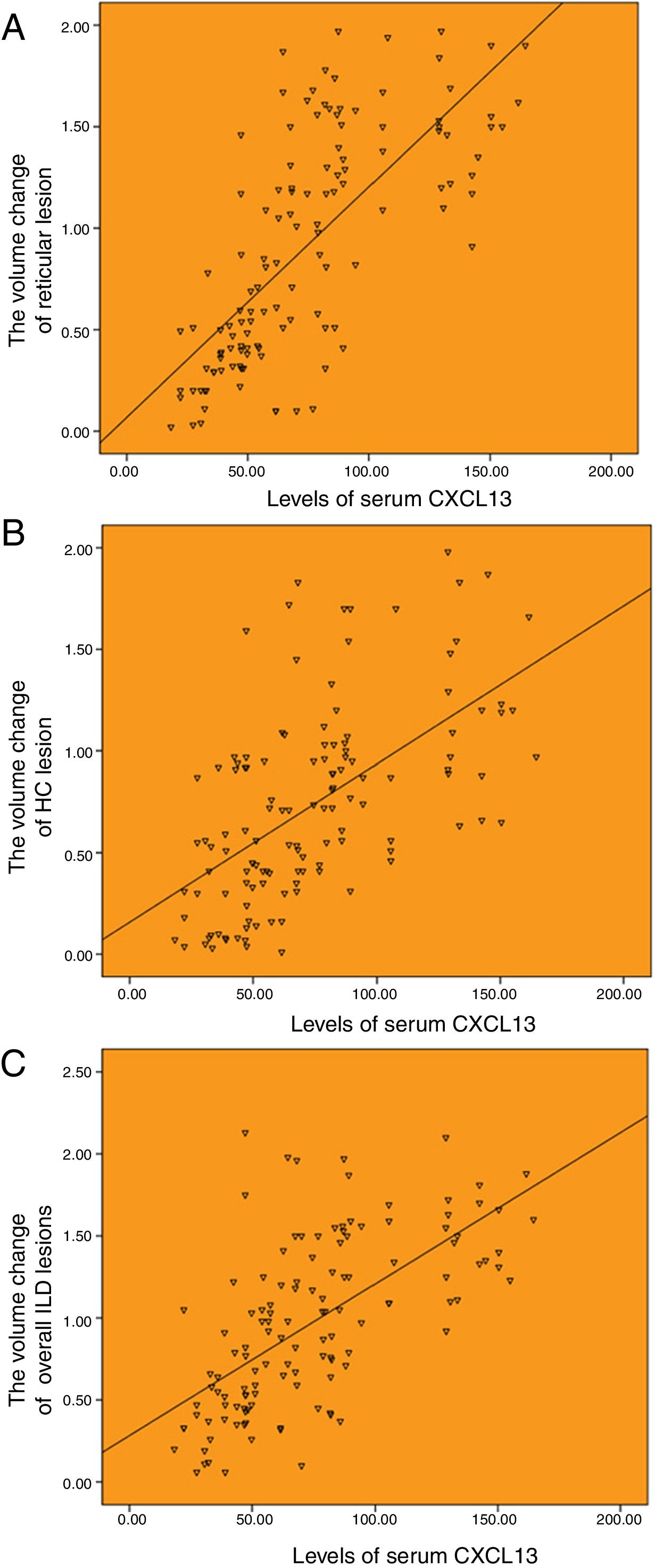
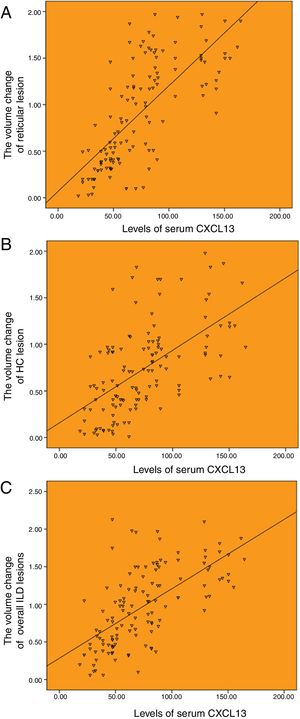
The serum CXCL13 baseline level was correlated with the changes of HRCT lesions. The serum level of CXCL13 was highly correlated with the annual increased volume of reticulation, and the annual increased volume of overall ILD lesions, with the correlation coefficients of r=0.720 and r=0.637 (P<.001), respectively (Fig. 4A and C). And moderately correlated with the annual increased volume of honeycombing, with a correlation coefficients of r=0.583 (P<.001; Fig. 4B).
Correlation between the baseline levels of serum CXCL13 and HRCT progression. (A) Correlation between the baseline levels of serum CXCL13 and annual increased volume of reticulation (r=0.720). (B) Correlation between the baseline levels of serum CXCL13 and annual increased volume of honeycombing (r=0.583). (C) Correlation between the baseline levels of serum CXCL13 and annual increased volume of totol ILD lesions (r=0.637).
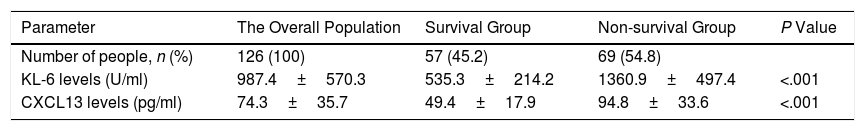
As shown in Table 5, the baseline levels of serum KL-6 and CXCL13 in the non-survival group were significantly higher than those in the survival group. Serum KL-6 levels were 1360.9±497.4U/ml and 535.3±214.2U/ml in the non-survival and survival groups, respectively. The serum CXCL13 levels were 94.8±33.6pg/ml and 49.4±17.9pg/ml in the non-survival and survival groups, respectively.
Comparison of Serum Levels of KL-6 and CXCL13 Between the Patients in Survival Group and Non-survival Groups.
| Parameter | The Overall Population | Survival Group | Non-survival Group | P Value |
|---|---|---|---|---|
| Number of people, n (%) | 126 (100) | 57 (45.2) | 69 (54.8) | |
| KL-6 levels (U/ml) | 987.4±570.3 | 535.3±214.2 | 1360.9±497.4 | <.001 |
| CXCL13 levels (pg/ml) | 74.3±35.7 | 49.4±17.9 | 94.8±33.6 | <.001 |
Serum markers, radiologic quantification and pulmonary function indexes have high warning value for the mortality risk of IPF patients.
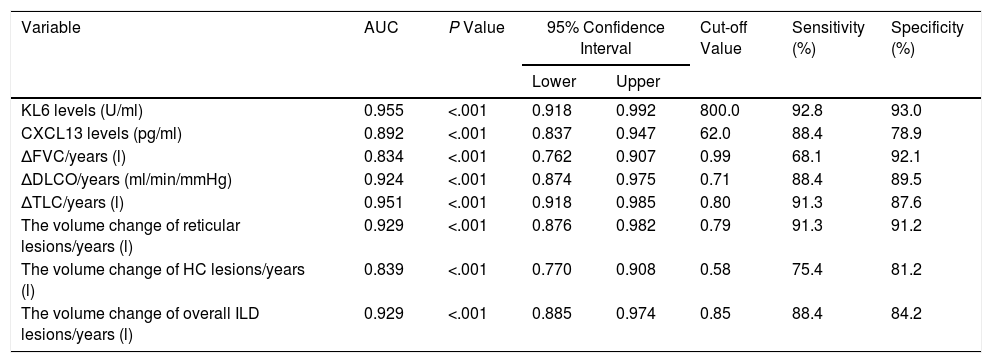
ROC curves were calculated to evaluate the sensitivity and specificity of the KL-6 and CXCL13. As shown in Table 6, the baseline level of serum KL-6 has a maximum area under the curve (AUC), and the other indicators also have good sensitivity and specificity (AUC>0.8). These results indicated that serum markers, radiologic quantification and pulmonary function indexes were of high warning value for the mortality risk of IPF patients. Further, we calculated that the cut-off values of serum KL-6 and serum CXCL13 were 800.0U/ml and 62.0pg/ml, respectively. To compare the effect of blood biomarkers, pulmonary function and quantitative monitoring by CAD system on IPF patient mortality, multivariate regression analysis was performed including parameters that were significantly associated with mortality, invariably, parameters that are considered obligatory (age and sex). After adjusted age and sex, there remained significantly associated with mortality (P<.05, Table S2).
The AUC and Cut-off Value of Serum Biomarkers and Clinical Features.
| Variable | AUC | P Value | 95% Confidence Interval | Cut-off Value | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| KL6 levels (U/ml) | 0.955 | <.001 | 0.918 | 0.992 | 800.0 | 92.8 | 93.0 |
| CXCL13 levels (pg/ml) | 0.892 | <.001 | 0.837 | 0.947 | 62.0 | 88.4 | 78.9 |
| ΔFVC/years (l) | 0.834 | <.001 | 0.762 | 0.907 | 0.99 | 68.1 | 92.1 |
| ΔDLCO/years (ml/min/mmHg) | 0.924 | <.001 | 0.874 | 0.975 | 0.71 | 88.4 | 89.5 |
| ΔTLC/years (l) | 0.951 | <.001 | 0.918 | 0.985 | 0.80 | 91.3 | 87.6 |
| The volume change of reticular lesions/years (l) | 0.929 | <.001 | 0.876 | 0.982 | 0.79 | 91.3 | 91.2 |
| The volume change of HC lesions/years (l) | 0.839 | <.001 | 0.770 | 0.908 | 0.58 | 75.4 | 81.2 |
| The volume change of overall ILD lesions/years (l) | 0.929 | <.001 | 0.885 | 0.974 | 0.85 | 88.4 | 84.2 |
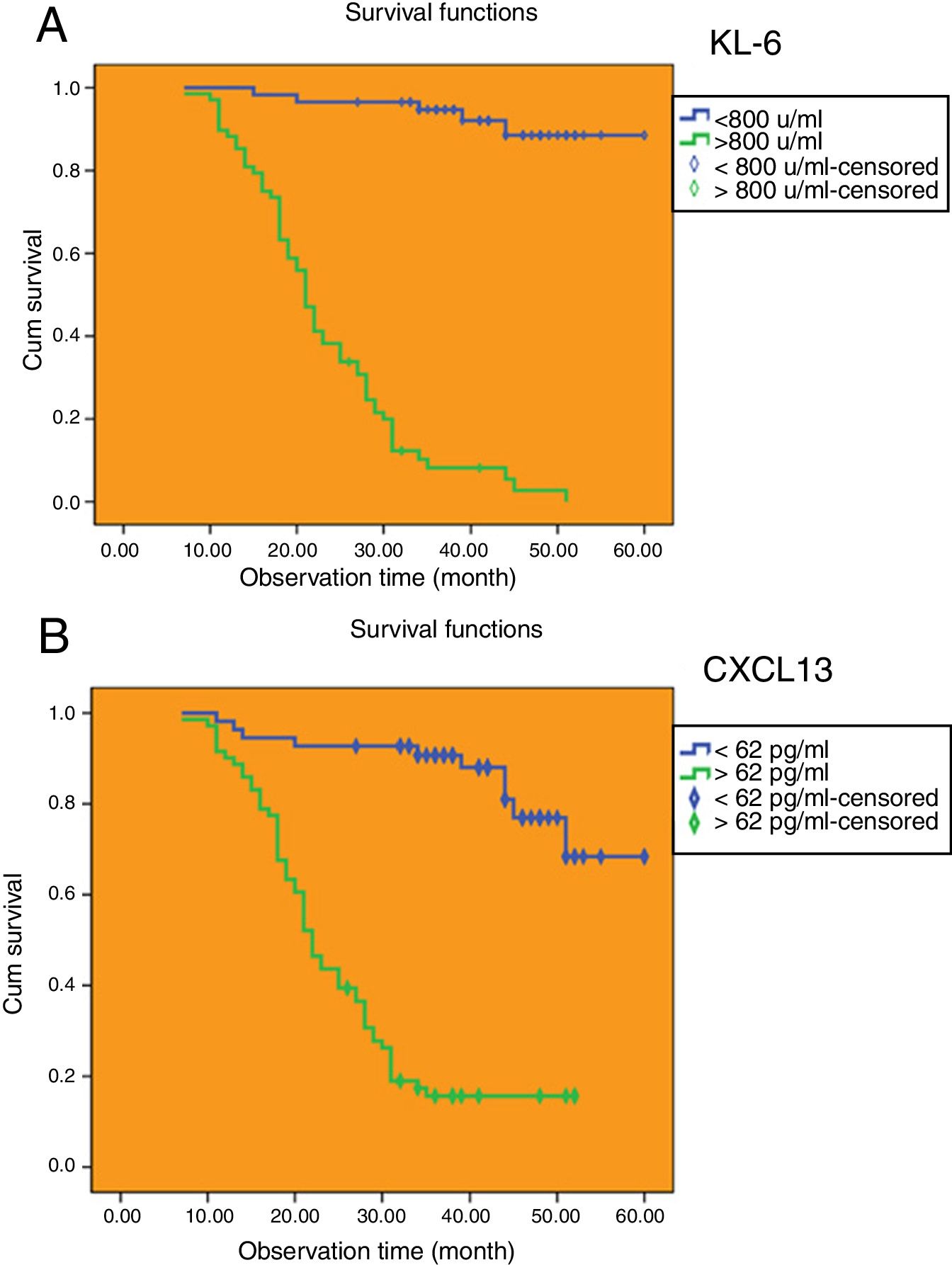
The cut-off values of serum KL-6 and CXCL13 have predictive value for median survival time of IPF patients.
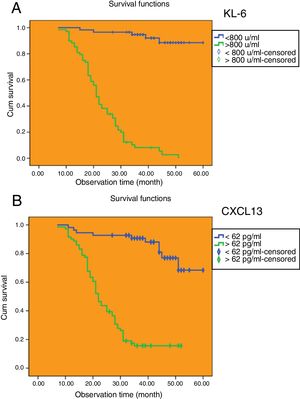
The Kaplan–Meier method was applied to calculate the survival time of IPF patients based on the cut-off values of serum KL-6 and serum CXCL13. As shown in Fig. 5A, there were 68 IPF patients (54.0%) whose serum KL-6 levels were higher than cut-off values, and the median survival time was 23.1 months (95% CI: 20.790, 25.503). Among the IPF patients whose serum KL-6 levels were lower than cut-off values, 58 (46.0%) had a median survival time of 56.9 months (95% CI: 54.334, 59.531). As shown in Fig. 5B, there were 71 IPF patients (56.3%) whose serum CXCL13 levels were higher than cut-off values, and the median survival time was 26.1 months (95% CI: 23.074, 29.066). And 55 IPF patients (43.7%) whose serum CXCL13 levels were lower than cut-off values, and the median survival time was 53.1 months (95% CI: 49.247, 56.958).
DiscussionIncreased circulating KL-6 levels in interstitial pneumonia are thought to be due to increased permeability of KL-6 following alveolar wall alveolar II cell regeneration and/or disruption of the air blood flow barrier in the affected lungs, which is associated with disease progression corresponding to HRCT. The pro-fibrogenic and anti-apoptotic effects of KL-6 increased the maximum effective concentrations of platelet-derived growth factor (PDGF), basic fibroblast growth factor (BFGF) and transforming growth factor-beta (TGF-β). These findings suggest that elevated levels of KL-6 in epithelial fluid may stimulate the fibrosis process of interstitial lung disease.4 CXCL13 is a chemokines produced by follicular dendritic cells, which recruit B cells to become secondary and tertiary lymphoid structures by binding to the homologous receptor CXCR5.14 Studies have shown that the expression of CXCL13 and clinical relevance in IPF patients are far greater than in chronic obstructive pulmonary disease (COPD) patients, indicating that the generation of this chemokines is not purely caused by the lung injury itself, but specific to the disease process of IPF.15 Increasing researches have indicated that the serum KL-6 and CXCL13 levels in IPF patients are higher than other lung diseases and interstitial lung diseases patients.5,15–17 At present study, we found that serum KL-6 and CXCL13 were better biomarkers for predicting the outcome of IPF patients, and their baseline levels were consistent with pulmonary function and the degree of changes in lesions quantified by CAD system, which could improve the accuracy of survival assessment for IPF patients.
As a prognostic predictor, we attempted to predict pulmonary function deterioration by measuring baseline levels of serum KL-6 and CXCL13 in IPF patients. Some research suggests that a high level of KL-6 is often associated with a significant decline in FVC (ΔFVC and %ΔFVC, respectively).18,19 Vuga et al. mentioned that serum CXCL13 level was weakly correlated with DLCO and FVC levels in recent studies, but this study did not compare the correlation between serum CXCL13 level and the dynamic changes in pulmonary function.15 Our study showed that the baseline level of serum KL-6 was highly correlated with the annual changes of pulmonary function (FVC, DLCO and TLC). The baseline level of serum CXCL13 was moderately or highly correlated with the annual changes of pulmonary function (FVC, DLCO and TLC), suggesting that IPF patients with higher serum kl-6 and CXCL13 baseline levels were more likely to have progressive deterioration of pulmonary function. Studies have shown that it is more accurate to reflect the prognosis of IPF according to the degree of reduced pulmonary function than histologic differences.20 Flaherty et al. demonstrated that short-term changes of FVC have a strong prediction for long-term survival of patients diagnosed with UIP, thus, baseline levels of serum KL-6 and CXCL13 may be markers of IPF progression.21
As HRCT can accurately reflect the pathological changes of IPF lesions, it is commonly used as a non-invasive assessment method for patients with diffuse infiltrative pulmonary disease.22 Sakamoto et al. reported that the correlation between KL-6 level and HRCT abnormal fibrosis progression in fibrous NSIP patients was statistically significant.23 However, there are few studies on the correlation between serum CXCL13 level and imaging.
In previous studies, we have found that the reticular lesions and HC lesions corresponding to fibrosis are the most important predictors for the prognosis of IPF patients, and the detection of clinical progression of overall ILD lesions is an important approach to reduce mortality. Therefore, our single-center prospective cohort study assessed the correlation between baseline levels of serum KL-6 and CXCL13 and HRCT disease dynamics.
Our study showed that the extent of volume change of the reticular lesions and overall ILD lesions was highly correlated with the baseline levels of serum KL-6 and CXCL13. And the volume change of HC lesions was highly correlated with the baseline level of serum KL-6, and moderately correlated with the baseline level of serum CXCL13. The high correlation between the serum KL-6 and CXCL13 baseline levels of IPF patients and the progression of HRCT lesions may reflect the severity of pulmonary fibrosis in advance and predict disease progression. Combined with the quantitative evaluation of HRCT lesions by CAD system, it is helpful to accurately select IPF patients with rapid progression clinically.
The heterogeneous characteristics of IPF make the survival time of patients have great individual differences.24 Recent studies have found that serum KL-6 and CXCL13 are associated with IPF patient's survival. In a prospective study involving 152 patients with idiopathic interstitial pneumonia (including IPF), patients with serum KL-6 level >1000U/ml had worse prognosis than patients with lower serum KL-6 level.25 In addition, Yokoyama et al. also reported that in a retrospective study of 27 IPF patients, the median survival time of patients with serum KL-6 level>1000U/ml was significantly reduced.19 The CXCL13 can be used as a biomarker for the prognosis of IPF patients.15,17 IPF patients with high levels of CXCL13 are often associated with pulmonary hypertension and respiratory failure.15 In our study, patients who died during five years of observation had significantly higher baseline levels of serum KL-6 and CXCL13 than those who survived. ROC curve analysis confirmed that the serum KL-6 baseline level was higher than the cut-off value 800.0U/ml and the serum CXCL13 baseline level was higher than the cut-off value 62.0pg/ml and had high sensitivity and specificity for the prediction of mortality risk. In addition, we found that the serum markers (including KL-6 and CXCL13), pulmonary function (including FVC, DLCO and TLC) and HRCT evaluation (including reticular lesions, HC lesions, and overall ILD lesions) have good sensitivity and specificity for the determination of survival rate (AUC>0.8). Among them, KL-6 has the maximum area under the curve (AUC 0.955, P<.001, 95% CI: 0.918, 0.941), so it has a strong predictive value for the poor prognosis of IPF patients.
Some scholars proposed that among the IPF patients with stable condition, serum KL-6>1000U/ml indicated poor prognosis.19,25 Hamai et al. reported that 1040U/ml was used as the cut-off value of serum KL-6 of 65 IPF patients, and the 5-year survival rate of the patients in the high level group was poor.16 Currently, no study has clearly shown the optimal cut-off value of CXCL13 for the survival of IPF patients, but the high level of CXCL13 is correlated with the low survival time.15,17 In our study, 800.0U/ml and 62.0pg/ml were used as the cut-off value of serum KL-6 and CXCL13, respectively. The difference in KL-6 cut-off value between our study and other studies may be related to the group selection bias, treatment differences and different sample sizes, prospective and large sample size of IPF study can further verify our experimental results. Following the kaplan–Meier method was used to estimate the survival rate, and it was found that the survival time between the survival group and non-survival group was statistically significant. The median survival time of patients with low level KL-6 was over 56 months, while patients with high level KL-6 were only 23 months. Median survival was more than 53 months for patients with low levels of CXCL13, while patients with high level CXCL13 were only 26 months. We research showed that the baseline levels of serum KL-6 and CXCL13 had a good correlation for lifetime prediction. Our results are different from other studies on cut-off value of KL-6, which may be related to the selection bias, treatment difference and sample size of the enrolled population. Prospective IPF studies with large sample size can further verify our experimental results.
ConclusionIn conclusion, measurement of baseline levels of specific serum markers KL-6 and CXCL13, radiologic quantification, and longitudinal follow-up and analysis of pulmonary function can all predict poor prognosis, such as disease deterioration and mortality risk in IPF patients, and also can assist in stratified management of IPF patients and selection of clinical strategies. As a non-invasive marker, its clinical value and its association with long-term survival rate of patients still need to be confirmed by further studies on multi-center and expanded number of cases.
Conflict of InterestThe authors declare that they have no conflicts of interest.
This work was supported by grants from National Health and Family Planning Commission of Sichuan Province (17PJ029).