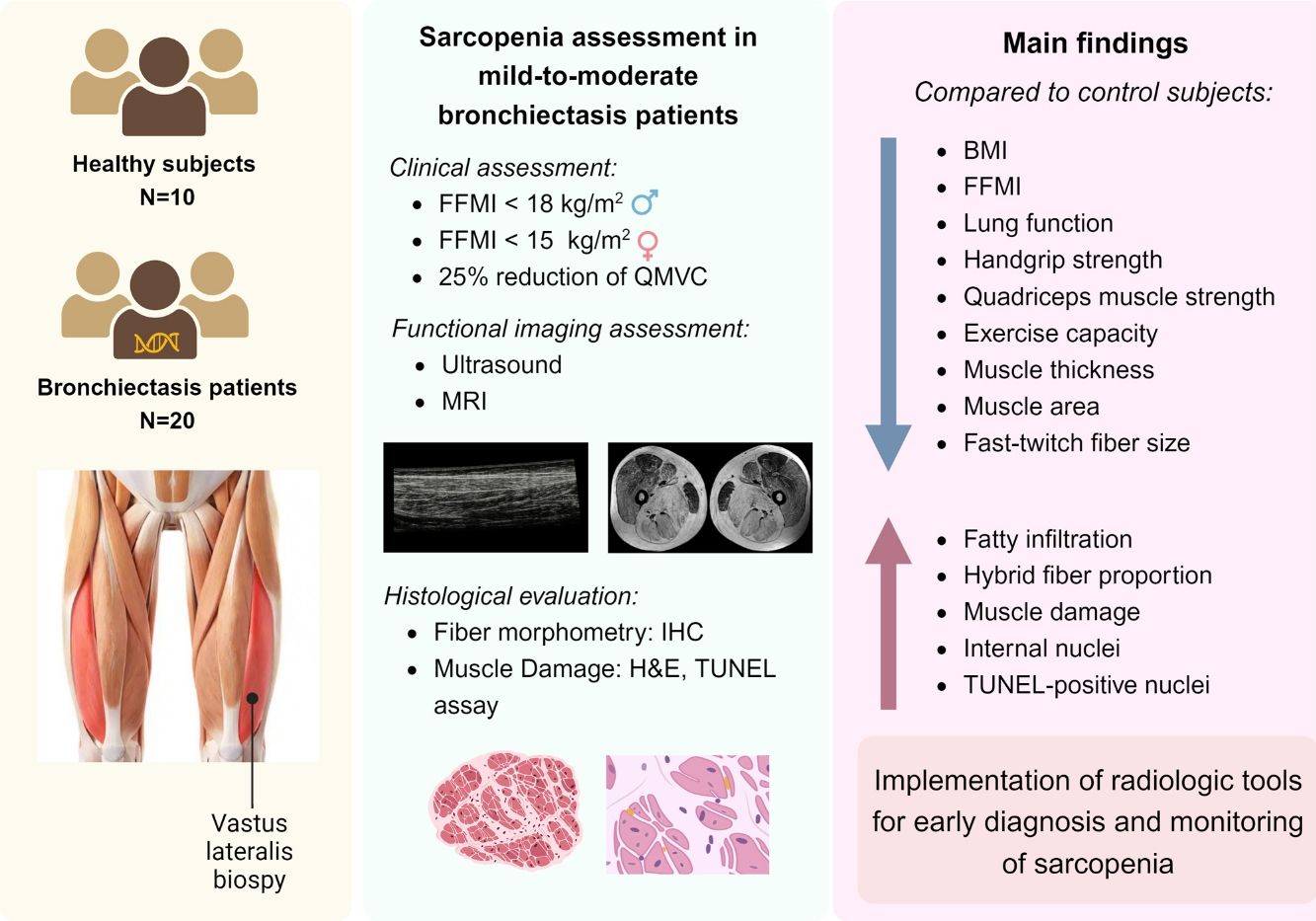
Bronchiectasis is a complex lung disease with poorly studied systemic manifestations. Patients with bronchiectasis-associated sarcopenia exhibit a specific differential profile of functional muscle phenotype (vastus lateralis, VL), which may be analyzed using imaging (ultrasound and magnetic resonance imaging, MRI).
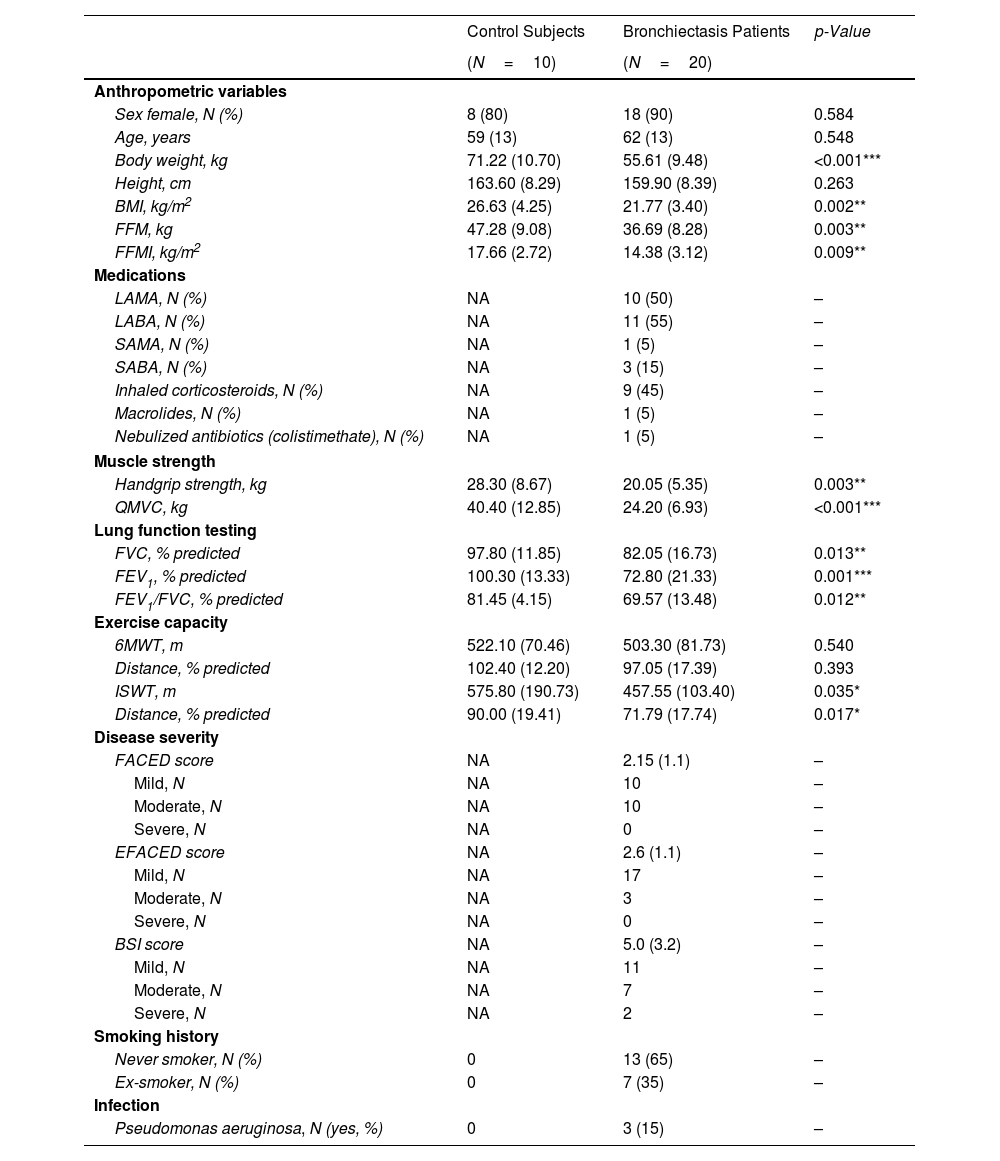
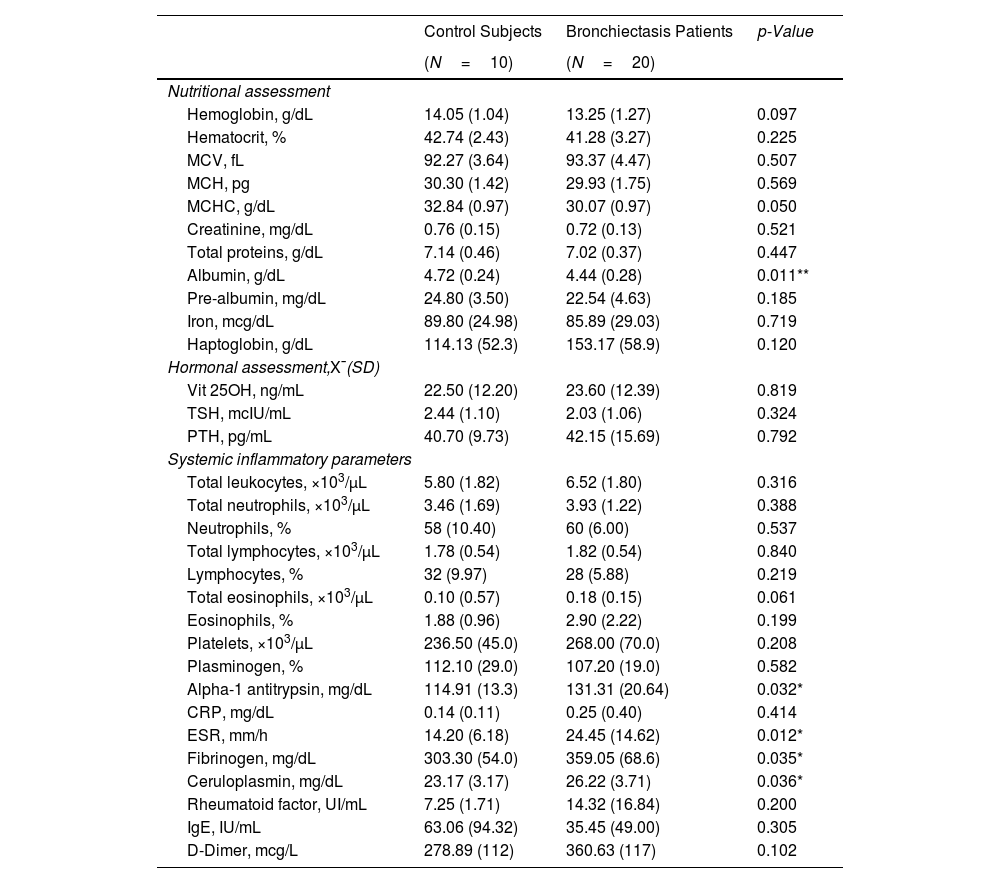
MethodsUltrasound and MRI were used to explore functional imaging parameters in quadriceps of 20 patients with stable bronchiectasis and 10 healthy controls. In muscle specimens (open biopsy procedures), muscle phenotype (fiber morphometry and structural abnormalities, immunohistochemistry) was also evaluated. Patients and controls were clinically and functionally evaluated.
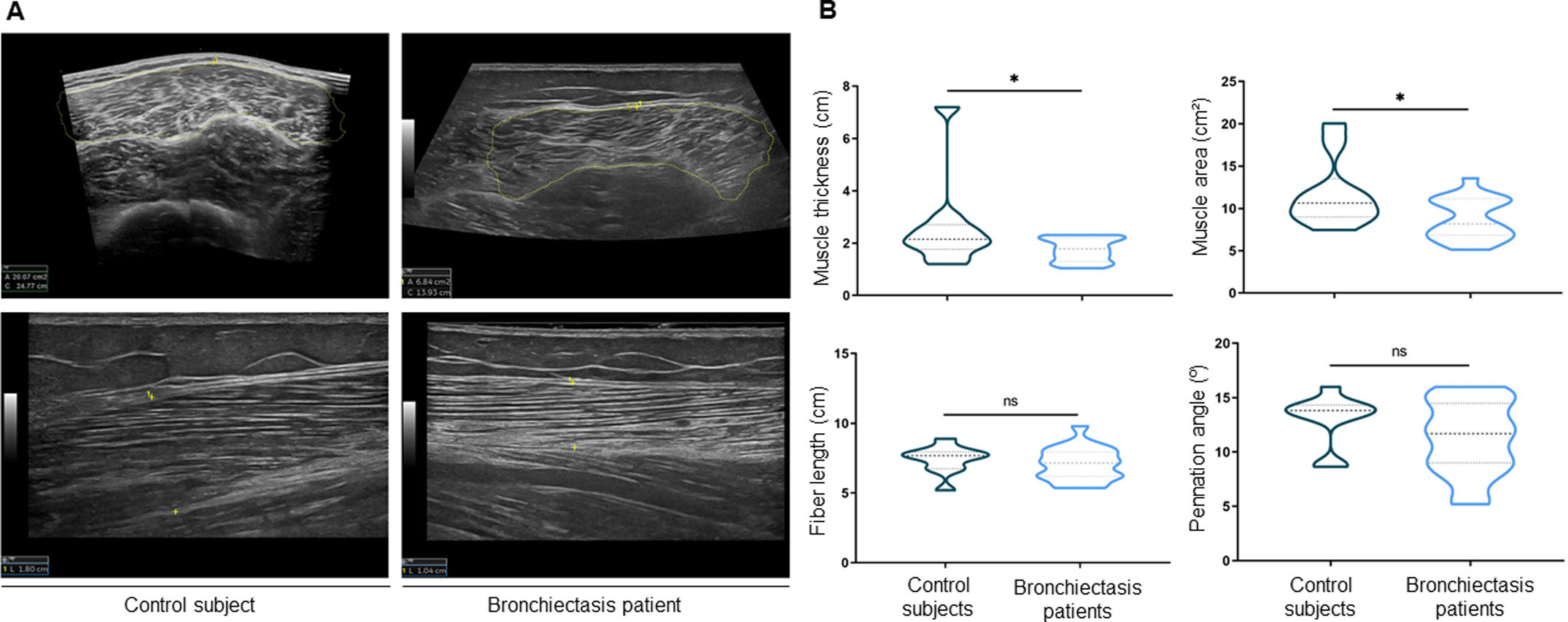
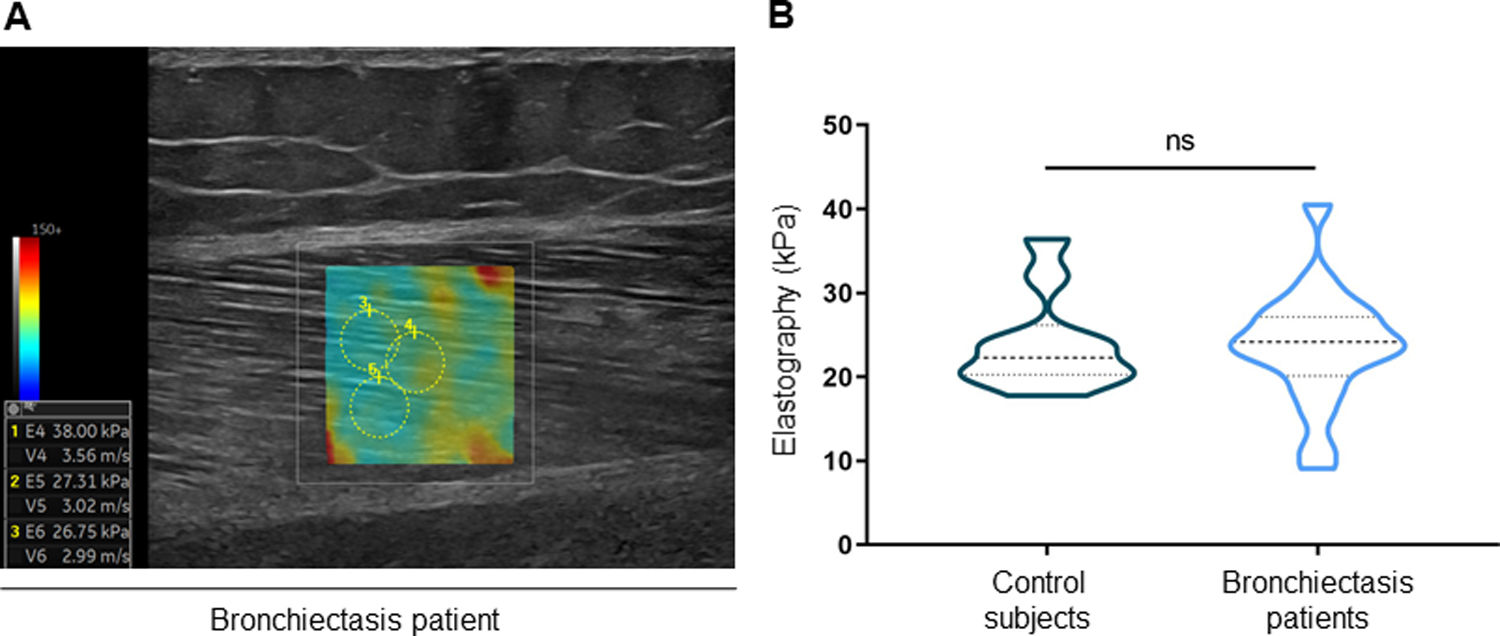
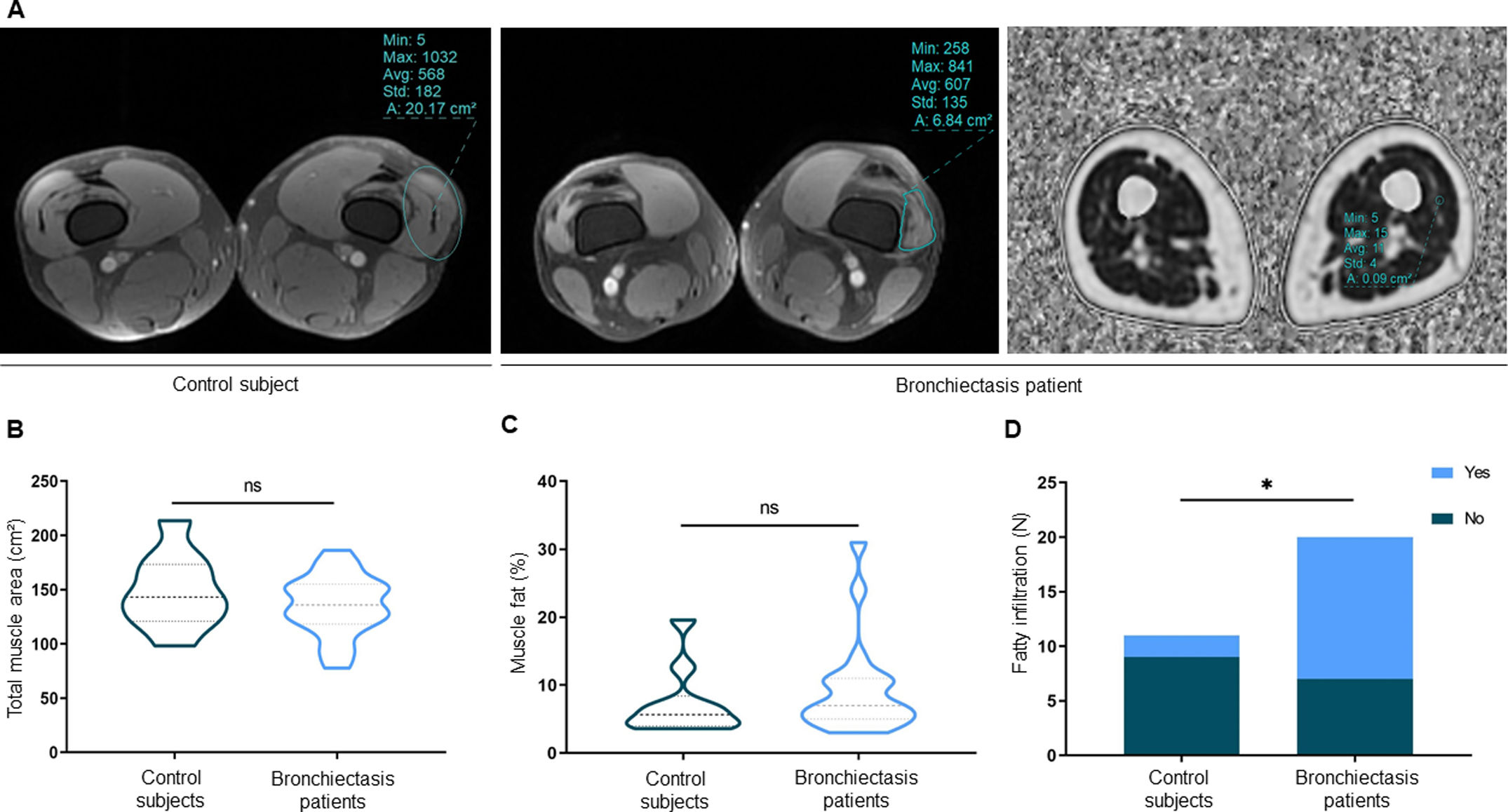
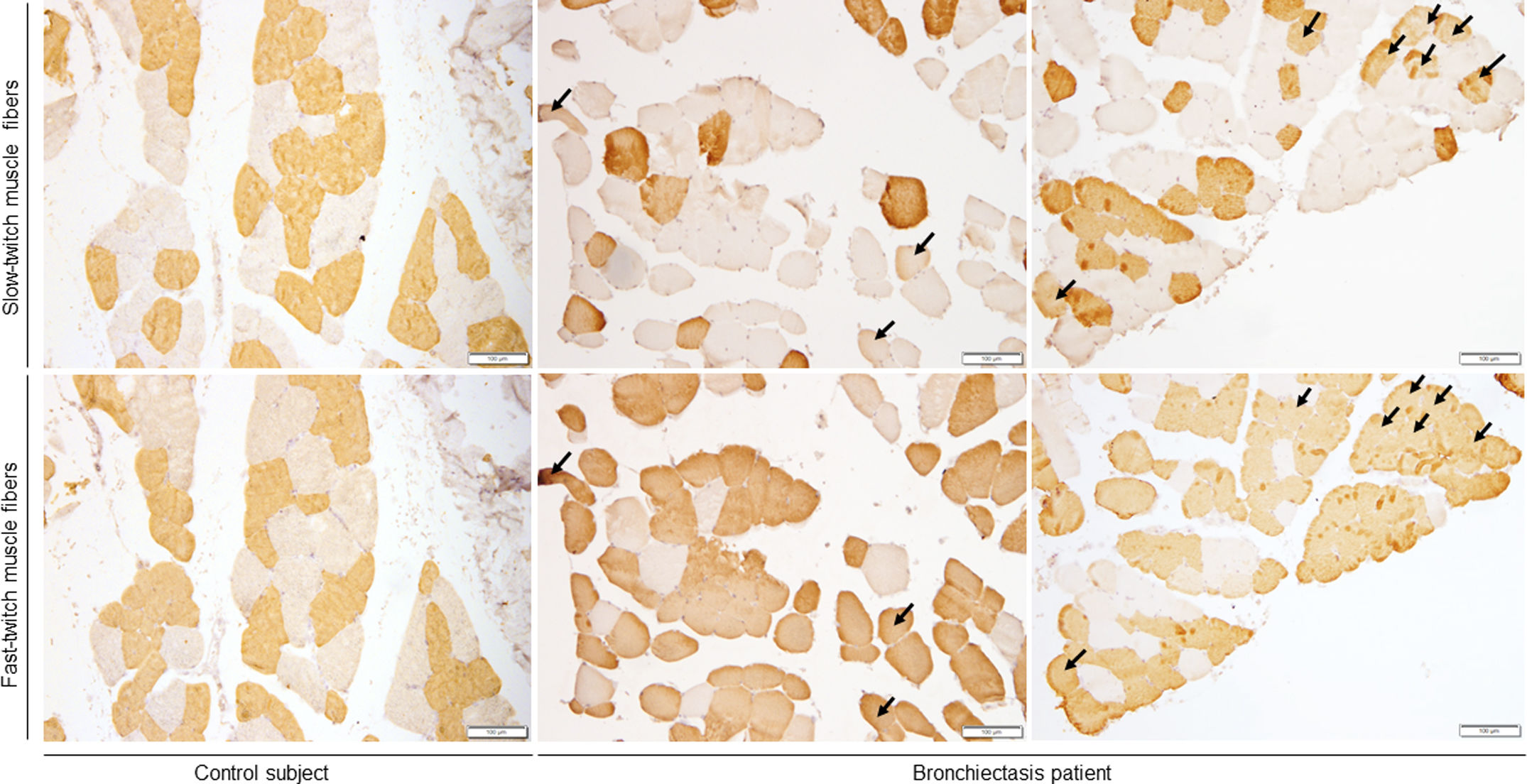
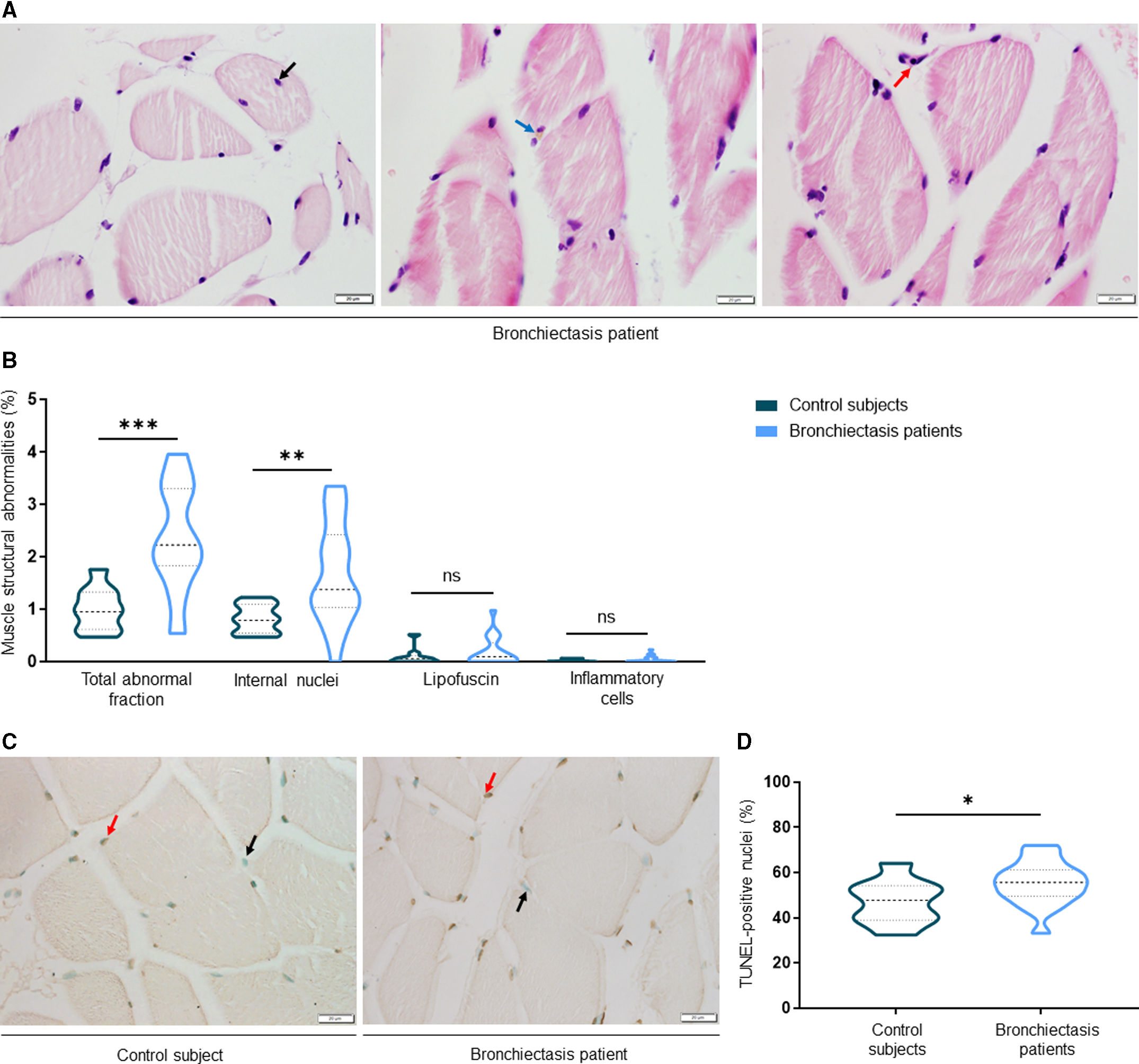
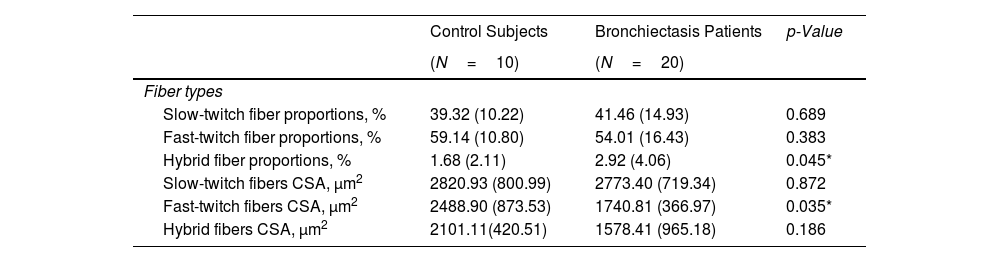
ResultsIn muscles of patients compared to controls, a significant decline in body composition parameters (BMI and FFMI), muscle function (upper and lower limbs), lung function, and exercise capacity was detected, ultrasonography revealed decreased muscle thickness and area, while MRI demonstrated increased fat infiltration, which positively correlated with the bronchiectasis severity scores. Structural parameters (proportions of hybrid fibers, internal nuclei, abnormal fibers, and apoptotic nuclei) were significantly greater in the VL of patients than in controls and inversely correlated with quadriceps muscle function and exercise capacity in the former.
ConclusionsIn patients with stable mild-to-moderate bronchiectasis, sarcopenia was clinically evidenced through the significant reduction in muscle mass and upper and lower limb muscle function. Non-invasive ultrasound and MRI techniques showed that features of muscle quality architecture and fat infiltration are hallmarks of bronchiectasis-associated sarcopenia. Functional radiological tools should be implemented in clinical settings to early diagnose and monitor sarcopenia in these patients.