Pulmonary function tests are vital for diagnosing lung diseases, assessing treatment responses, and monitoring respiratory health. Recent updates to interpretive standards by the European Respiratory and American Thoracic Societies (ERS/ATS) in 2022 introduced significant changes compared to the 2005 standards. They include incorporating lung volume measurements, non-specific and mixed disorders, introducing z-scores for functional abnormality assessment, reducing severity categories from five to three, and revising criteria for positive bronchodilator responses.
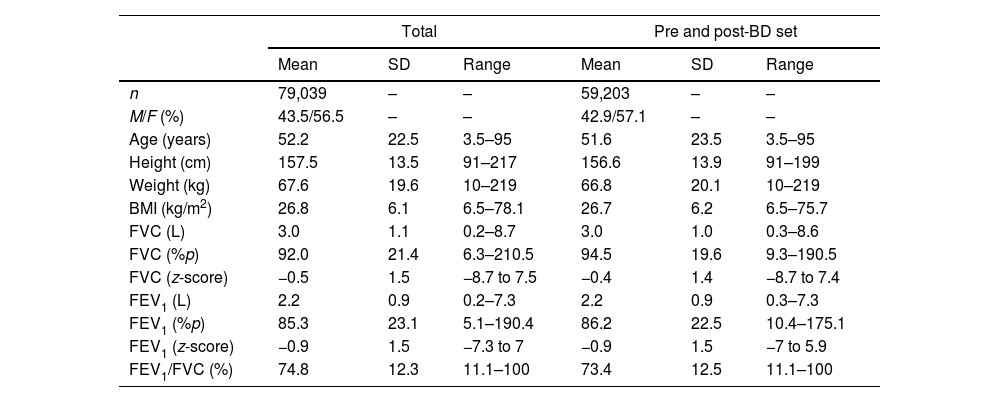
MethodsWe conducted a retrospective, multi-center study across four centers using spirometric data spanning from 2002 to 2022. We categorized spirometry results using both the 2005 and 2022 ATS/ERS standards and calculated predicted values following the GLI 2012 equation (Caucasian subset).
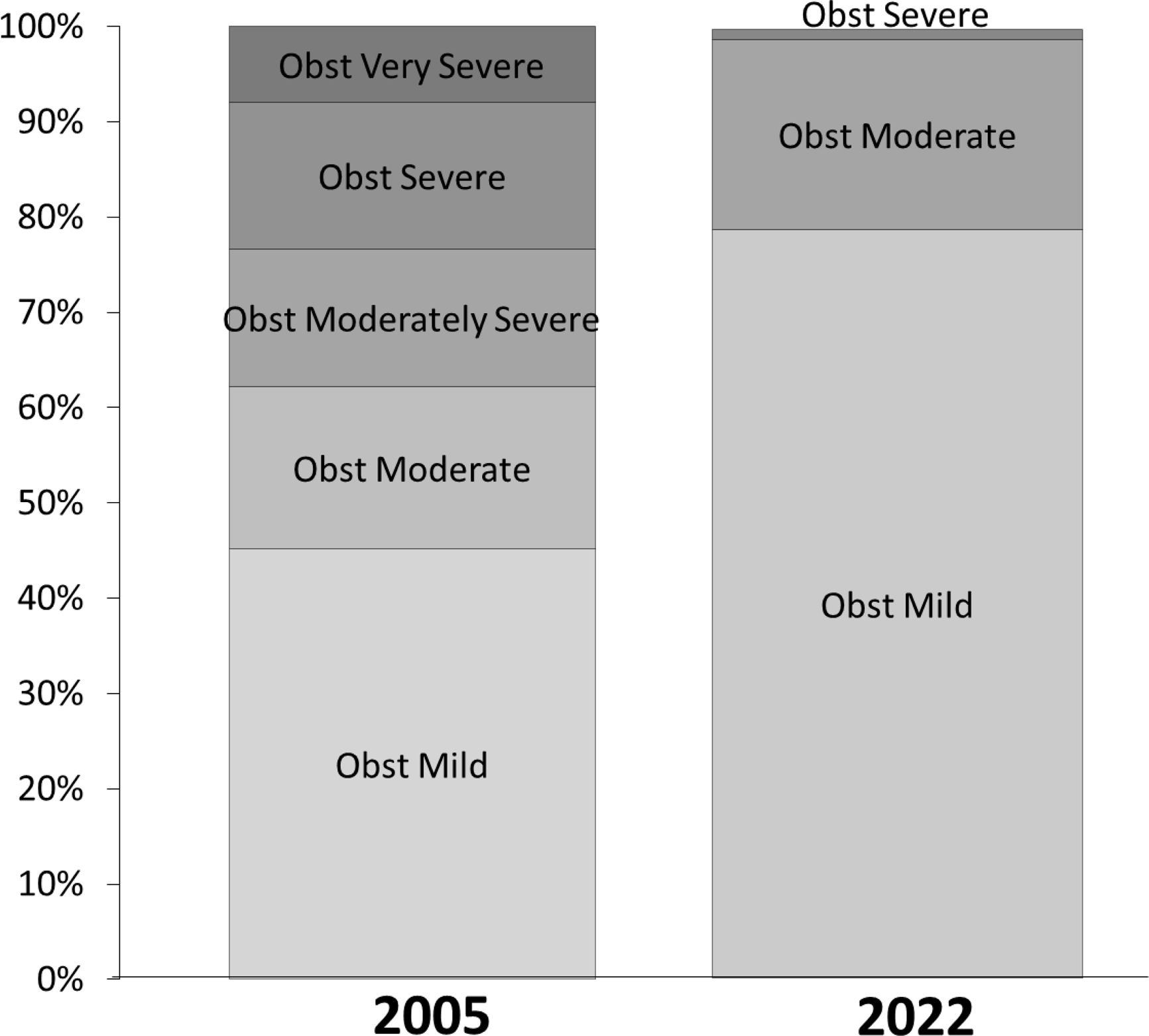
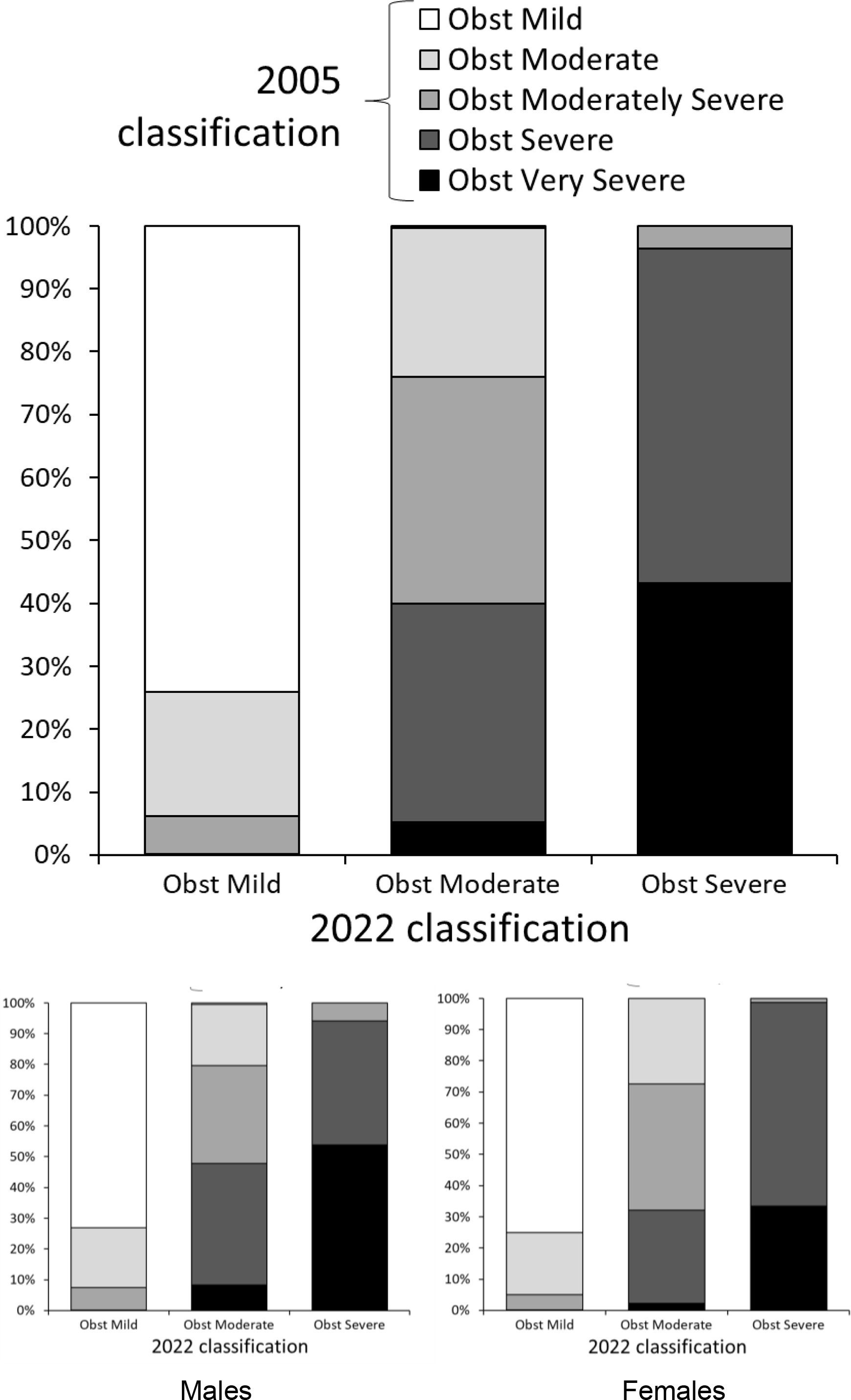
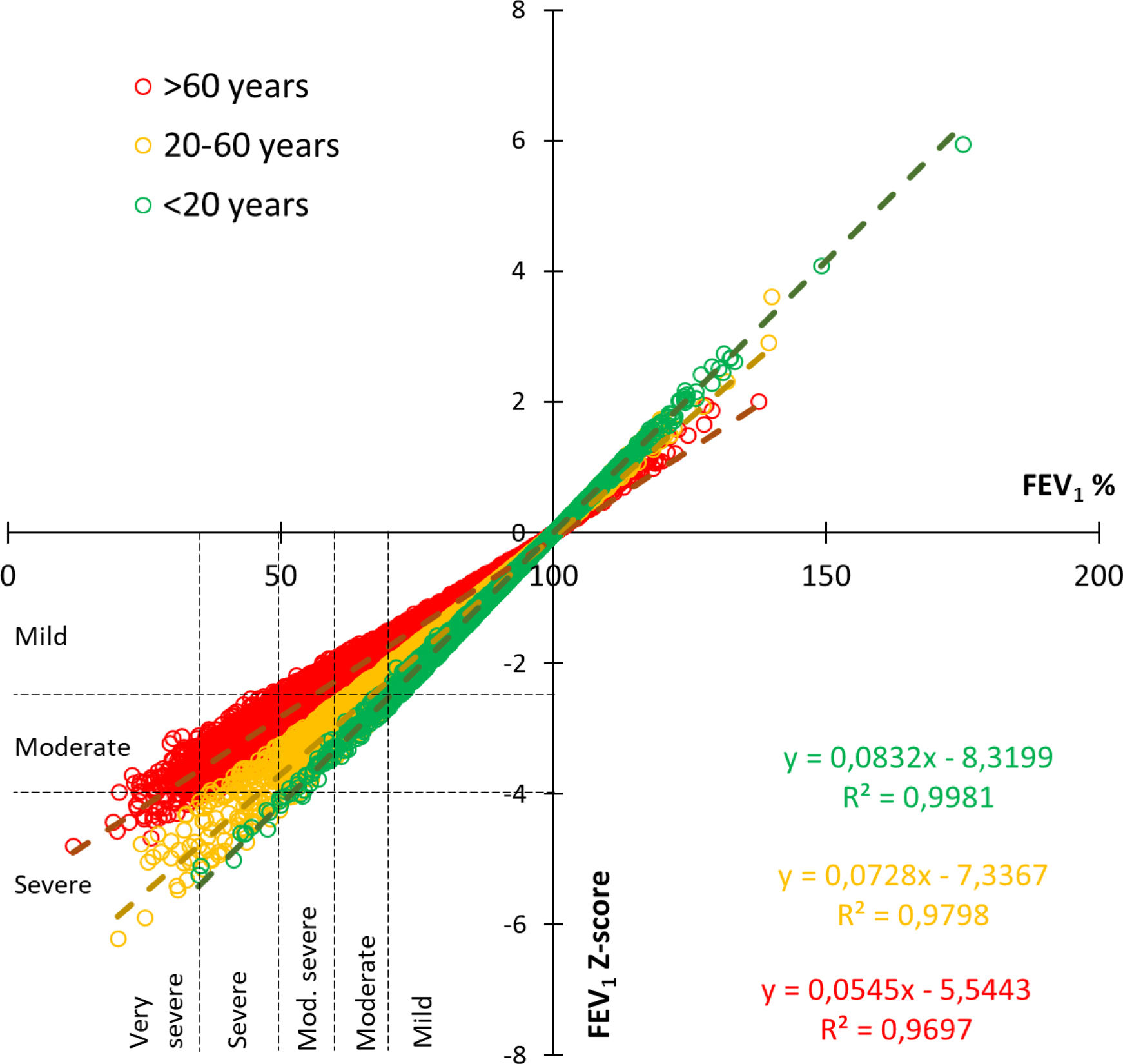
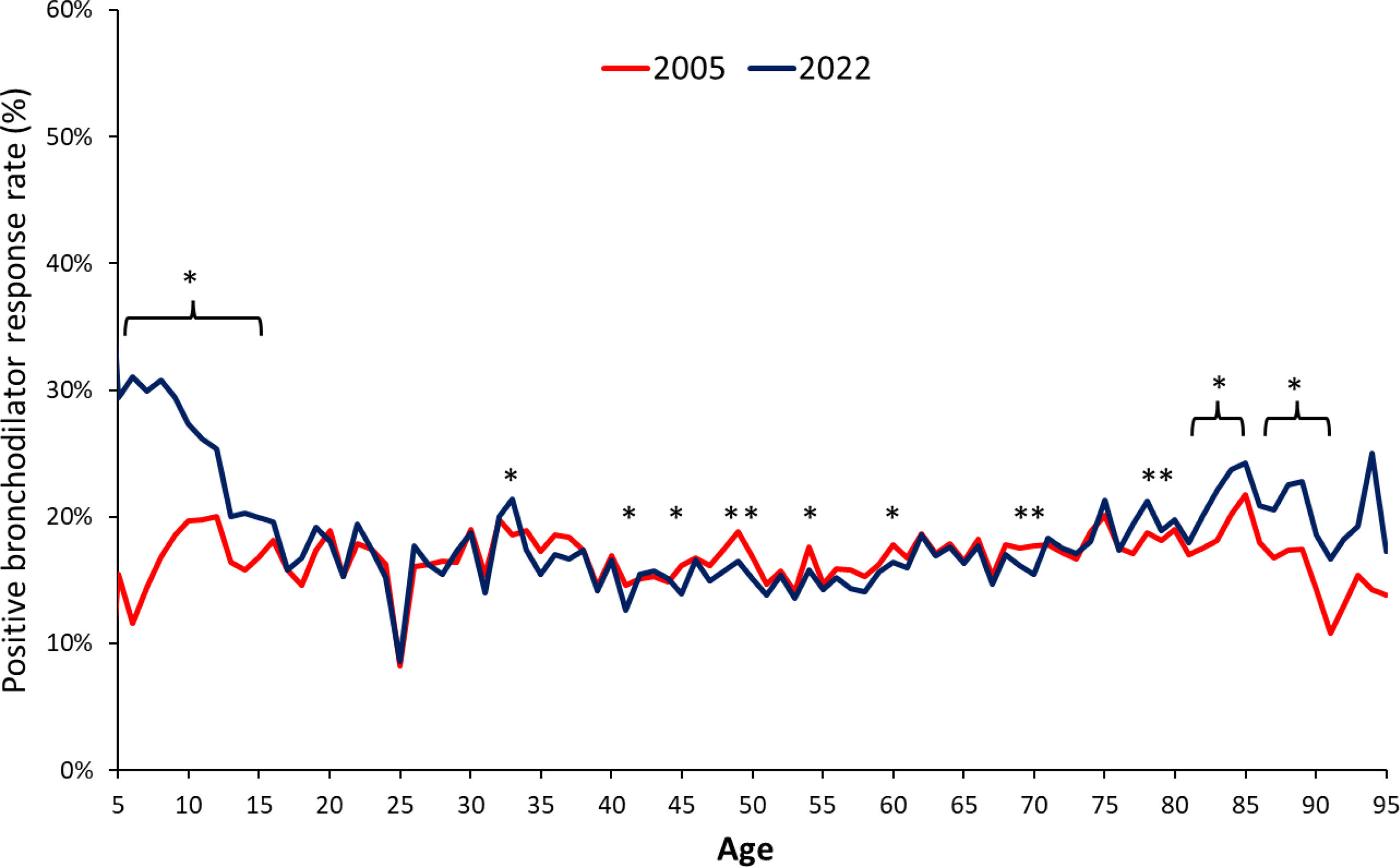
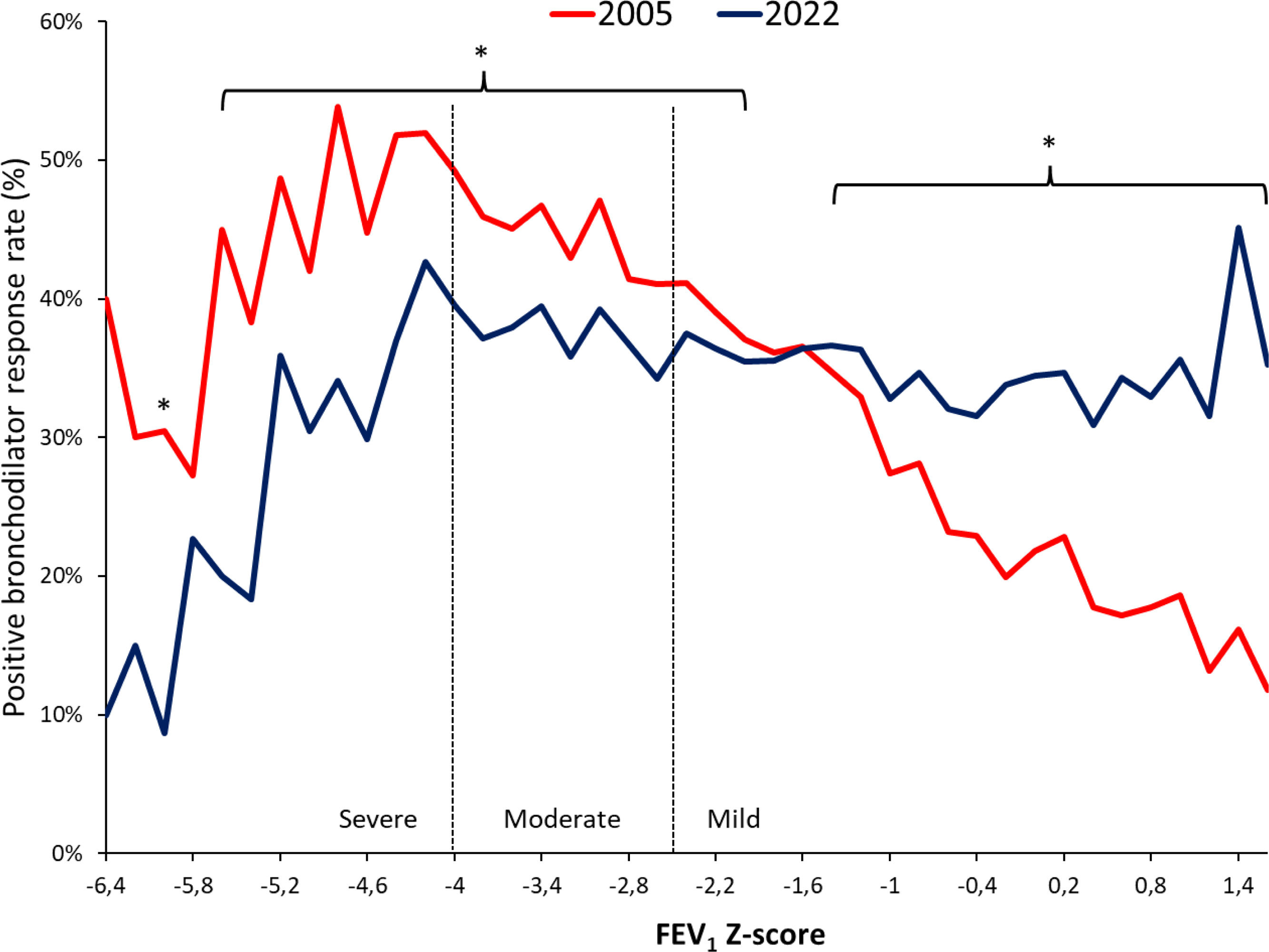
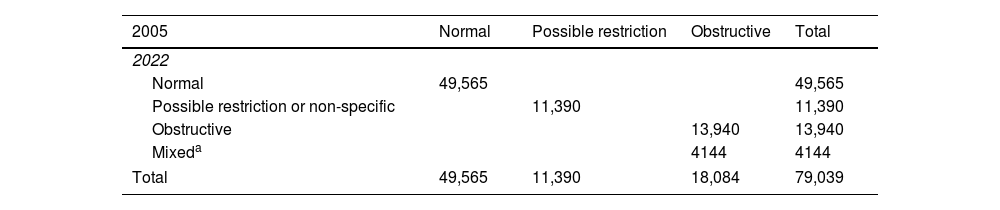
ResultsAmong 79,039 subjects, we observed that 23% shifted from an obstructive diagnosis under the 2005 standard to a mixed pattern diagnosis under the 2022 standard, necessitating lung volume assessments. In the evaluation of bronchodilator responses among 59,203 tests, 12.3% of those initially classified as responders were reclassified as non-responders with the new standards. We found variations in severity categorization across age groups, with older patients tending to receive milder severity classifications and younger individuals receiving greater severity classifications under the 2022 standards.
ConclusionsThe 2022 document emphasizes early lung volume assessment, potentially leading to increased utilization of more complex tests. Furthermore, the bronchodilator response was predominant in extreme age groups and among individuals with milder spirometric impairments. This shift may impact treatment decisions, potentially initiating medication in milder cases and de-escalating treatment in more severe cases.