Bronchiectasis (BE) is the third most common chronic inflammatory airway disease, following asthma and chronic obstructive pulmonary disease (COPD). It is a heterogeneous and complex condition, and recent efforts have focused on identifying phenotypes, endotypes, and treatable traits that may guide precision and personalized treatments.1 However, our current understanding of these aspects in BE is considerably less extensive than in asthma or COPD, leading to many uncertainties regarding the most effective therapeutic regimens.2,3 Blood eosinophil count (BEC) is a biomarker of T2-high inflammation that has been widely accepted as a treatable trait, useful for guiding treatments such as inhaled corticosteroids or biologic therapies in asthma and COPD.4 Recent research suggests that BEC in a stable state is associated with clinical outcomes in BE5 and may predict responses to inhaled corticosteroids.6,7 However, there is considerably less knowledge regarding the significance of BEC during exacerbations of BE. The objective of the present study was to investigate whether BEC during severe BE exacerbations requiring hospitalization is related to significant clinical outcomes, independent of BEC during stable phases.
This study involved a retrospective analysis of data from consecutive patients included in a registry of all individuals seen at the BE clinic of the Pulmonology Service of a second-level university hospital. Due to the retrospective design, all patients seen at the BE clinic and included in the registry were eligible for inclusion. Approval was obtained from the hospital and the ethical committee (CEIC Galicia, registry: 2015/63). Patients who were admitted to the hospital after their first visit (index date) to the BE clinic were included in the study. The primary outcome variable was hospital readmission following this initial admission. The following variables were recorded for all patients on the index date: age, sex, FEV1%, FEV1/FVC%, oxygen saturation (SpO2), eFACED (exacerbations, FEV1, age, bronchial infection by Pseudomonas aeruginosa [PA], radiological extension, dyspnea) score; isolation of PA during the previous year; isolation of any potential pathogenic microorganism (PPM) during the previous year; chronic bronchial infection at the index date; non-age-adjusted Charlson comorbidity index; BEC; polymorphonuclear leukocyte (PM) count; platelet count; C-reactive protein (CRP); and treatment at the index date (bronchodilators, inhaled corticosteroids, inhaled antibiotics, azithromycin). Variables obtained during the first hospital admission after the index date included: BEC, PM and platelet count as measured in the emergency room before treatment initiation; CRP; presence of purulent sputum; chest X-ray findings of pulmonary infiltrates; respiratory failure; hemoptysis; fever; isolation of any PPM during admission; isolation of PA during admission; and treatment with systemic steroids or antibiotics during admission. Differences between study groups were assessed using Student's t-test or the Chi-square test, as appropriate. A univariable logistic regression analysis was performed with hospital readmission after the first hospital admission as the dependent variable. All variables with a p-value of less than or around 0.1 (i.e., <0.2) were included in a multivariable Cox proportional hazards analysis, with readmission as the dependent variable. The correlation between BEC at the index date and during admission was evaluated using the Pearson correlation coefficient. Receiver-operating characteristic (ROC) analysis was conducted to identify the BEC cut-off value that best correlated with readmission risk, and Kaplan–Meier curves were generated using this value. Significance was defined as a p-value of <0.05. Additional details on the statistical analysis are provided in the supplemental file.
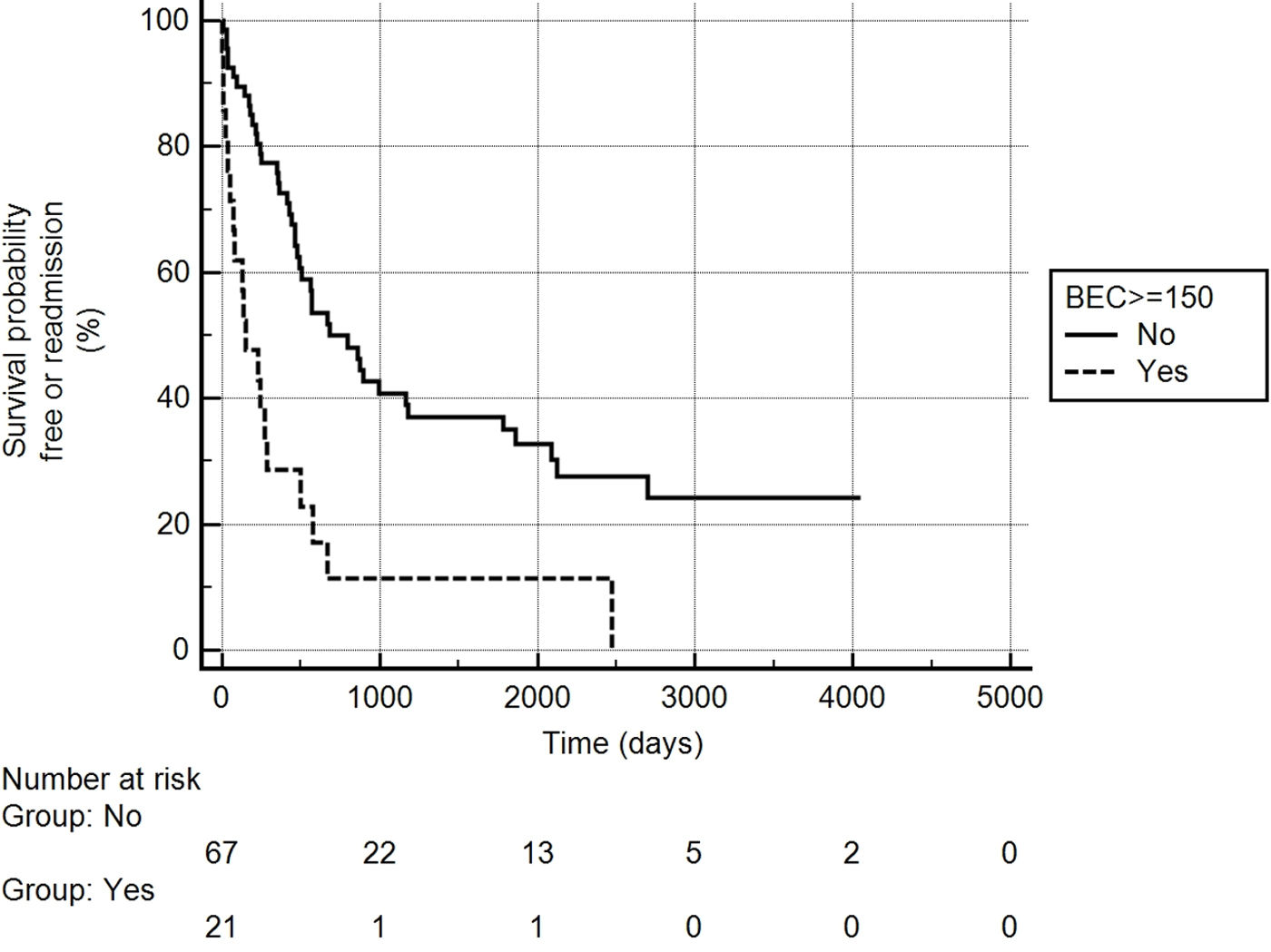
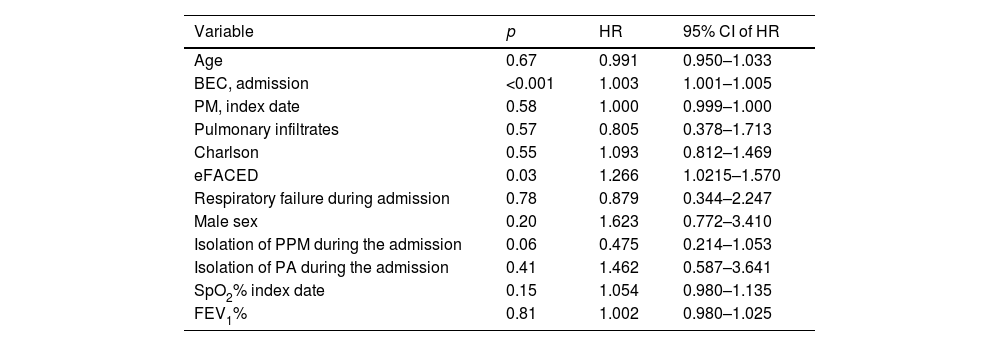
A total of 180 patients were included in the registry. Of these, 89 (49%) experienced at least one severe exacerbation after the index date. The most common etiologies of BE were idiopathic, COPD, and post-infectious. The etiologies are detailed in the supplemental file (Table S1). Differences between patients who experienced an exacerbation after the index date and those who did not are presented in the supplemental file (Table S2). One patient died during admission and was excluded; the remaining 88 patients were included in the analysis. Among these, 62 (70.4%) were readmitted due to exacerbation, with a median of 350 days between admissions (interquartile range: 130–665). Table S3 in the supplemental file presents the results of the univariable logistic regression analysis, with hospital readmission as the dependent variable. In this analysis, BEC on admission (but not at the index date), the Charlson index, eFACED score, and respiratory failure during admission significantly correlated with the risk of readmission. Table 1 shows the results of the multivariable Cox proportional hazards analysis, using variables selected from the univariable analysis as independent variables and readmission as the dependent variable. The eFACED score and BEC on admission were both associated with an increased risk of readmission, with the hazard ratio of 1.003 per unit increase in BEC (cells/mm3). ROC analysis (see supplemental file) identified a cut-off value of ≥150eosinophils/mm3 as providing the best combination of sensitivity and specificity for predicting readmission risk. Fig. 1 illustrates the Kaplan–Meier curves for the risk of readmission based on this cut-off value. There was no significant correlation between BEC at the index date and during admission (R=0.07, p=0.49).
Results of the multivariable Cox proportional hazards analysis using readmission as the dependent variable.
| Variable | p | HR | 95% CI of HR |
|---|---|---|---|
| Age | 0.67 | 0.991 | 0.950–1.033 |
| BEC, admission | <0.001 | 1.003 | 1.001–1.005 |
| PM, index date | 0.58 | 1.000 | 0.999–1.000 |
| Pulmonary infiltrates | 0.57 | 0.805 | 0.378–1.713 |
| Charlson | 0.55 | 1.093 | 0.812–1.469 |
| eFACED | 0.03 | 1.266 | 1.0215–1.570 |
| Respiratory failure during admission | 0.78 | 0.879 | 0.344–2.247 |
| Male sex | 0.20 | 1.623 | 0.772–3.410 |
| Isolation of PPM during the admission | 0.06 | 0.475 | 0.214–1.053 |
| Isolation of PA during the admission | 0.41 | 1.462 | 0.587–3.641 |
| SpO2% index date | 0.15 | 1.054 | 0.980–1.135 |
| FEV1% | 0.81 | 1.002 | 0.980–1.025 |
BEC: blood eosinophil count; PM: polymorphonuclear leukocyte count; eFACED: exacerbations, FEV1, age, colonization by Pseudomonas aeruginosa, radiological extension, dyspnea score; SpO2: oxygen pulse saturation; FEV1: forced expiratory value in the first second.
Several studies have analyzed the inflammatory profile of BE during stable states, frequently using BEC as a surrogate for airway inflammation. As seen in other chronic respiratory diseases, this biomarker has been associated with clinical outcomes. Specifically, both eosinopenia and eosinophilia have been linked to a higher risk of exacerbations.6 It remains unclear whether the inflammatory profile during BE exacerbations correlates with that observed during stable phases of the disease or whether BEC during exacerbations has independent clinical significance. To our knowledge, only one study has assessed the clinical significance of BEC during BE exacerbations, finding that patients with higher BEC experienced longer hospital stays and incurred higher hospitalization costs.8 In other inflammatory airway diseases, such as COPD, BEC in stable states correlates to some extent with BEC during exacerbations, although the correlation is not perfect and does not allow precise predictions of the inflammatory profile of future exacerbations.9,10 Indeed, the inflammatory profile of COPD exacerbations is heterogeneous,11 and BEC during these episodes has both prognostic12 and therapeutic implications, potentially guiding the treatment of the exacerbation itself.13,14 Hypothetically, the inflammatory profile during COPD exacerbations could also inform maintenance therapy, although this has not been fully demonstrated.15 Therefore, it is of interest to study the potential significance of BEC during BE exacerbations. The results of the present study suggest that BEC during a severe BE exacerbation is correlated, independently of the BEC during stable phases and other clinical and analytical variables, with a significant clinical outcome: the risk of future readmission. This finding underscores the need for further research to explore whether the exacerbation phenotype of BE, specifically BEC during exacerbations, may constitute a distinct, independent treatable trait. Hypothetically, eosinophilia during BE exacerbations could guide decisions on whether to treat the exacerbation with systemic steroids or whether to add inhaled steroids as maintenance therapy to prevent future eosinophilic exacerbations. However, this remains entirely speculative at present.
Several limitations of the study must be acknowledged. We opted to use readmission risk as the primary outcome rather than more robust outcomes like mortality due to the small sample size, which resulted in few mortality events and limited the power of the analysis. Additionally, being a single-center, retrospective study, the results should be considered preliminary and require validation through further prospective, multicenter studies. The low sample size may have limited the detection of some associations due to a potential type II error. Moreover, steady-state BEC was measured only once, and it is known that this biomarker can vary significantly over time.16 Consequently, the relationship between steady-state and exacerbation BEC may not be entirely reliable.
In conclusion, higher BEC during hospitalization for a BE exacerbation is associated with an increased risk of readmission. Further studies are needed to clarify other possible prognostic and therapeutic implications of this biomarker during BE exacerbations.
Artificial intelligence involvementNo AI tool or software has been used to produce this manuscript.
FundingThis manuscript has received no funding.
Conflicts of interestThe authors state that they have no conflicts of interest related to this manuscript.