While the beneficial effects of physical fitness on general health are well-documented, the specific relationship between different types of physical fitness, particularly cardiorespiratory fitness (CRF) and muscular endurance fitness (MEF), and lung function in physically active young adults remains less explored.
ObjectiveThis study investigated the relationship between CRF and MEF, and their correlation with lung function in physically active young adults.
MethodsThis cross-sectional study involved a cohort of 1227 physically active young adults without lung diseases. Lung function was assessed using FEV1, FVC, and FEV1/FVC measurements. The 3000-m run was used to assess CRF, and the 2-min push-up and sit-up tests were used to assess MEF. Multivariable linear regression analysis was used to evaluate the relationships between these fitness measures and lung function, adjusting for potential covariates.
ResultsEnhanced CRF was associated with superior FEV1 and FVC after adjusting for covariates (β=−.078, p=.015 for FEV1; β=−.086, p=.009 for FVC). Push-ups were positively associated with FEV1 (β=.102, p=.014), but not with FVC. In contrast, sit-ups showed no significant correlation with lung function in the fully adjusted model.
ConclusionThe study demonstrated a clear association between improved physical fitness and better lung function in physically active young adults, with various exercises showing distinct associations with lung metrics. Notably, push-ups were particularly associated with higher FEV1. A future prospective study is necessary to determine whether routine exercises, such as push-ups, might lead to greater lung function.
Pulmonary function is a fundamental aspect of respiratory health, achieving its highest levels in young adulthood, around the ages of 20–25.1 Tobacco smoke exposure is a well-documented major risk factor for “impaired respiratory health”—a condition that can progress to chronic lung disease due to diminished lung growth and function.2 Furthermore, recent research also suggests associations between impaired respiratory health and factors such as air pollution, obesity, and low physical fitness levels.2,3 A reduction in lung function not only limits exercise capacity but also increases the risk of respiratory and cardiovascular diseases, lowers health-related quality of life (HRQoL), and results in increased respiratory hospitalizations, mortality, and healthcare demands.4–6 Therefore, it is crucial to identify and address these modifiable risk factors to preserve and enhance lung function.
Physical fitness serves as a critical indicator of health outcomes and influences long-term health.7 Studies have evaluated physical fitness through cardiorespiratory fitness (CRF) and muscular fitness, using various tests to measure them either separately or together.8 Research suggests that enhanced aerobic fitness in childhood and adolescence plays a significant role in lung development, leading to improvements in FEV1 and FVC from ages 9 to 26.9 Additionally, young adults with higher baseline CRF levels, as measured by treadmill tests, showed reduced declines in FEV1 and FVC over 20 years, according to the Coronary Artery Risk Development in Young Adults (CARDIA) study.10 While these findings suggest that CRF contributes to lung development and may help protect against future declines in lung function, the relatively small effect sizes reported indicate that the impact of fitness on lung function requires further clarification. Furthermore, the causal relationship between improved lung function and gains in fitness remains lacking. In the cohort, when the data was analyzed with lung function as the independent variable and fitness as the dependent variable, it yielded similar results.9 These results suggest a bidirectional relationship: individuals with superior lung function might be predisposed to higher physical fitness levels.
Muscular fitness, characterized by both muscular endurance (repeated force exertion) and muscular strength (single maximal effort), is essential for sustained activity and peak performance.11 In children and adolescents, higher muscular fitness correlates with lower obesity rates, diminished cardiometabolic risks, and improved HRQoL.12,13 A meta-analysis highlighted that strong muscular fitness during these early years is associated with less body fat and better cardiometabolic health later in life.14 Additionally, a recent study has found a positive link between muscular strength (measured by handgrip strength and chair stand tests) and lung function in a community general population.15 However, the exact relationship between CRF, muscular endurance fitness (MEF), and their impact on lung function, especially among physically active individuals, is not fully understood.
The Cardiorespiratory Fitness and Health In Armed Forces (CHIEF) Lung Study aims to evaluate the association between CRF (time for a 3000-m run) and MEF (2-min push-ups and 2-min sit-ups numbers) with pulmonary function, and to clarify their individual impacts on the respiratory health of active young adults. We hypothesize that superior CRF and MEF levels are positively associated with pulmonary function, with each type of fitness making a unique correlation.
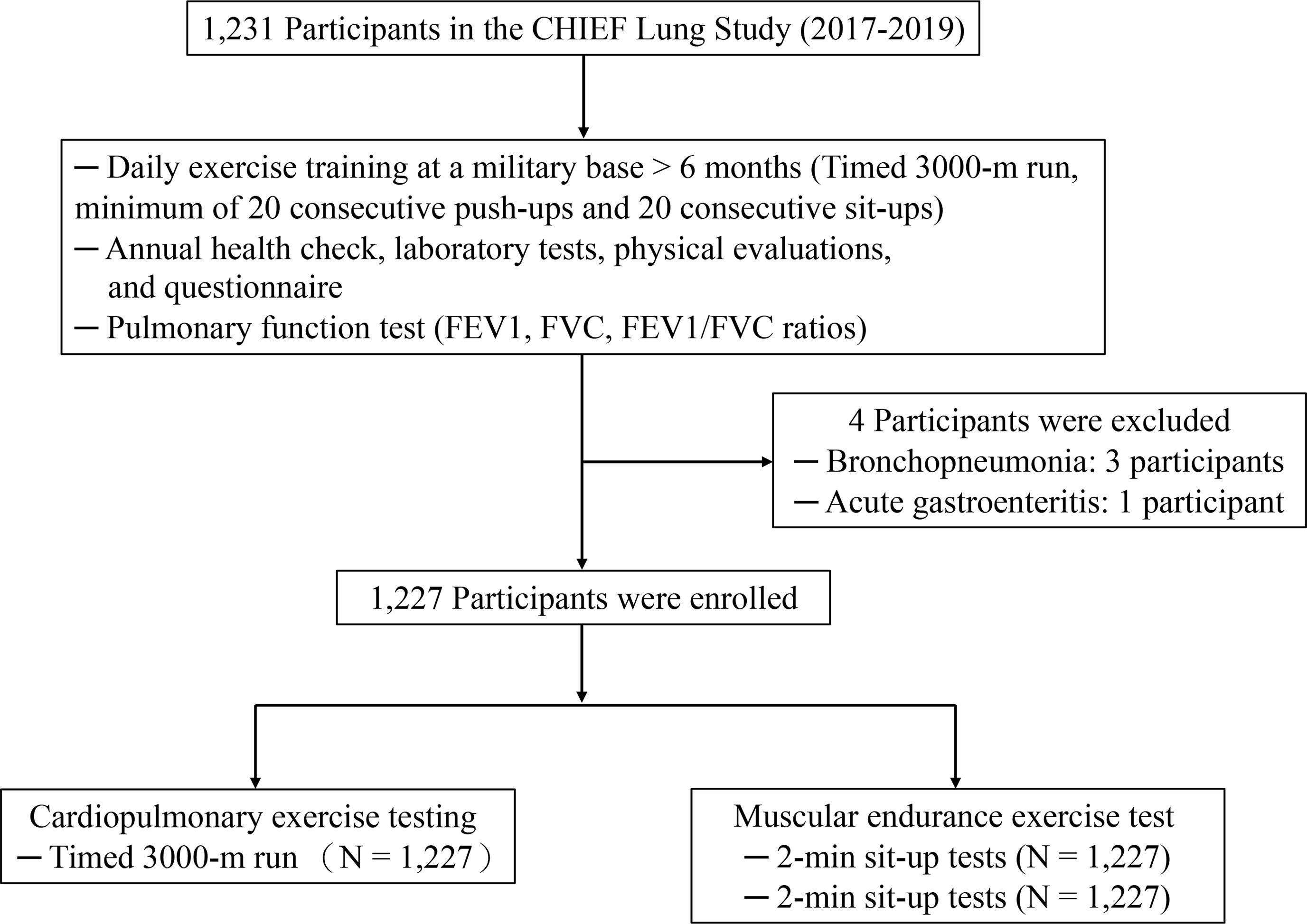
MethodsStudy populationThe CHIEF Lung Study meticulously assessed lung function in 1231 military recruits, both male and female, aged 19–38, across Taiwan from 2017 to 2019 (Fig. 1). Participants underwent medical exams for military enlistment, with no history of pulmonary tuberculosis, chronic lung conditions (e.g., lung abscess, emphysema, asthma), diaphragmatic or pleural disorders, chest wall deformities, or surgeries for pneumothorax or segmentectomy. They participated in a rigorous daily exercise regimen at a military base for six months, focusing on muscular endurance training and cardiovascular fitness. The regimen required all military personnel to complete a 3000-m run twice daily at 6:00AM and 4:00PM, led by the captain at a pace of 120m per minute on weekdays. Following each run, all participants were required to perform 20 consecutive push-ups and sit-ups within 30min. This training course lasted until the end of June. Before the mid-term physical test in July, all participants underwent an annual health checkup in June, which included laboratory tests and physical evaluations. They also filled out a questionnaire about substance use habits, such as cigarette smoking (active and former/never), alcohol consumption, and betel nut chewing at the Hualien Armed Forces General Hospital. Furthermore, participants underwent pulmonary function testing to assess their FEV1, FVC, and the ratio of FEV1/FVC before undergoing the annual military exercise tests. The study adhered to stringent ethical standards and was approved by the Institutional Review Board of Mennonite Christian Hospital in Hualien City (No. 16-05-008), ensuring that all participants provided informed consent.
Blood pressure and anthropometric measurementsBlood pressure (BP) was measured once on each participant's right arm after a 15-min rest, using an automatic device (FT201) via the oscillometric method. Mean Arterial Pressure (MAP) was calculated using the formula: MAP=diastolic BP+1/3 (systolic BP−diastolic BP). Anthropometric data, including waist circumference (WC), height, and weight, were recorded for standing participants, with body mass index (BMI) calculated as weight (kg) divided by height squared (m2).
MEF and CRF assessmentsMEF in participants was assessed using 2-min push-up and sit-up tests, adhering to protocols from previous studies.16 These evaluations were scheduled between 14:00 and 15:00 at the Military Physical Training and Testing Center, allowing for a flexible sequence. Performance was recorded on sponge pads with electronic or infrared sensors: sit-ups required elbow contact with thigh-mounted sensors, with hands near ears and feet fixed; push-ups demanded correct elbow motion and a straight body alignment, confirmed by sensors, disqualifying any touch outside of hands or toes before time. CRF was gauged through a 3000-m run, performed outdoors on level ground after an hour's rest from MEF tests. This field test, held outdoors on flat terrain at the Military Physical Training and Testing Center, was scheduled for 16:00, following an hour's rest after the muscular endurance tests. Participants wore standard sweat suits and carried no extra items.17 The entire run for each participant was video-recorded and monitored by eight military sports officers. The run was contingent upon certain environmental factors: the coefficient, determined by the product of the outdoor temperature (in Celsius) and the relative humidity (%) multiplied by 0.1, had to be below 40, and there should not have been any significant rainfall. The completion time of the 3000-m run was used to assess each participant's cardiorespiratory endurance capacity.
Pulmonary function assessmentLung function was measured with a Jaeger VyntusTM Pneumo spirometer as per the American Thoracic Society.18 Participants, seated with a nose clip, performed the test thrice for accurate FVC, FEV1, and FEV/FVC ratios, inhaling fully and exhaling rapidly for 6s. The best effort was recorded via a flow-volume curve. Lung function is expressed as Z-scores using the Global Lung Initiative reference equations, accounting for height, sex at birth, age, and Asian ethnicity. In addition, the predicted percentage of lung function was also presented.
Statistical analysesContinuous variables were presented as mean±standard deviation (SD) and categorical ones as frequencies and percentages (%). Descriptive statistics described demographics and clinical characteristics. The fitness data are presented as the median (interquartile range). Pearson's correlation and scatter plots with r and p values analyzed the relationship between pulmonary parameters and the performance of each physical test. Multivariable linear regression analysis was used to examine the associations between physical performance measurements and lung functions. The analysis was stratified into four distinct models, each designed to incrementally build upon the previous by integrating additional variables. Model 1 served as the baseline, adjusting for demographic and physiological factors including age, gender, height, weight, waist circumference, mean arterial pressure, and smoking status. Model 2 expanded on this by incorporating the 3000-m run time and the number of 2-min push-ups, aiming to assess their specific impacts. Model 3 followed a similar format but focused on the 3000-m run time and 2-min sit-ups. Finally, Model 4, the most comprehensive of the series, combined all the metrics from the previous models to provide a holistic view of the effects of combined physical activities on lung function. Results across these models were expressed through standardized beta coefficients, with significance levels noted for key findings (*p≤0.05, **p≤0.01). The p-interaction value was calculated to assess whether there are sex differences in the association between physical fitness and lung function. Analysis was performed using SPSS (version 29).
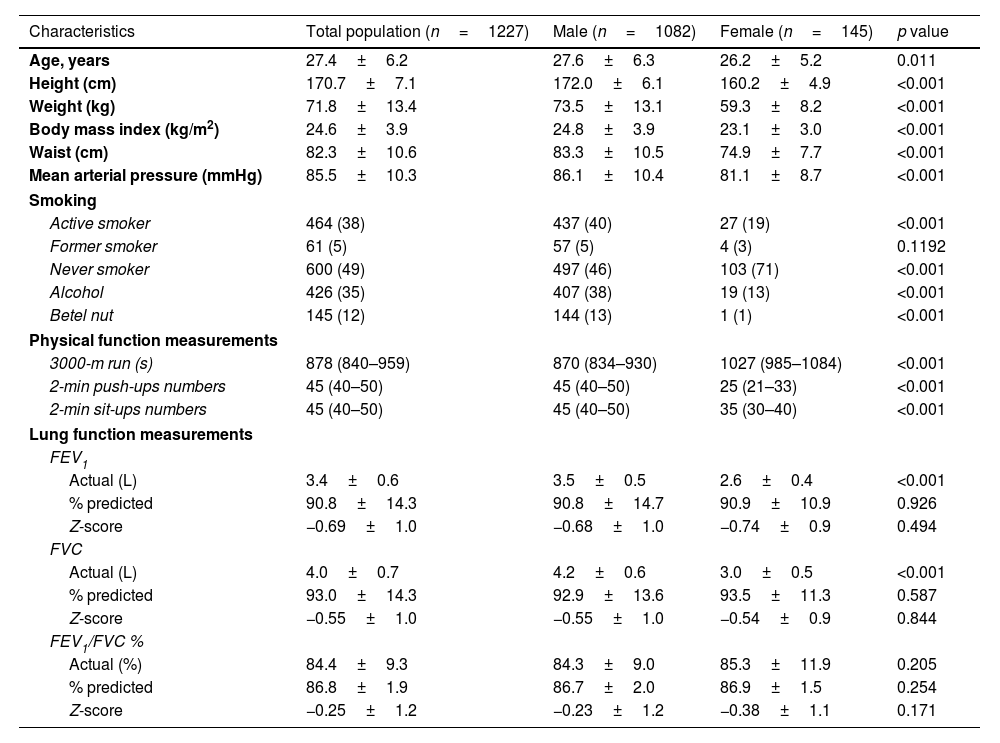
ResultsOut of the initial cohort, four were excluded due to acute illnesses, leaving 1227 participants in the final analysis. Clinical characteristics of participants are shown in Table 1. The participants, primarily male at 88.2%, had an average age of 27.4 years. They maintained a healthy physique with an average BMI of 24.6kg/m2, and a waist circumference of 82.3cm. Smoking status varied, with 38% currently smoking and 54% either former smokers or having never smoked. Regarding physical fitness, the participants completed a 3000-m run in an average time of 15min and 12s and demonstrated considerable strength and endurance by performing an average of 44.1 push-ups and 44.6 sit-ups within a two-minute timeframe for each exercise. In the study comparing clinical and physiological characteristics between sexes, notable differences were observed. Males were older, taller, and heavier than females, with correspondingly higher BMI and waist circumferences. Significant differences in smoking habits were evident, with a higher percentage of males being active or former smokers compared to females, where a majority were never smokers. Physical fitness assessments showed that males performed better in muscular endurance exercises like push-ups and sit-ups, and demonstrated superior cardiorespiratory fitness, evidenced by shorter times in the 3000-m run compared to females. Lung function measurements revealed higher absolute values of FEV1 and FVC, but no differences were observed in standardized lung function metrics such as predicted percentages and Z-scores.
Clinical characteristics of study population.
| Characteristics | Total population (n=1227) | Male (n=1082) | Female (n=145) | p value |
|---|---|---|---|---|
| Age, years | 27.4±6.2 | 27.6±6.3 | 26.2±5.2 | 0.011 |
| Height (cm) | 170.7±7.1 | 172.0±6.1 | 160.2±4.9 | <0.001 |
| Weight (kg) | 71.8±13.4 | 73.5±13.1 | 59.3±8.2 | <0.001 |
| Body mass index (kg/m2) | 24.6±3.9 | 24.8±3.9 | 23.1±3.0 | <0.001 |
| Waist (cm) | 82.3±10.6 | 83.3±10.5 | 74.9±7.7 | <0.001 |
| Mean arterial pressure (mmHg) | 85.5±10.3 | 86.1±10.4 | 81.1±8.7 | <0.001 |
| Smoking | ||||
| Active smoker | 464 (38) | 437 (40) | 27 (19) | <0.001 |
| Former smoker | 61 (5) | 57 (5) | 4 (3) | 0.1192 |
| Never smoker | 600 (49) | 497 (46) | 103 (71) | <0.001 |
| Alcohol | 426 (35) | 407 (38) | 19 (13) | <0.001 |
| Betel nut | 145 (12) | 144 (13) | 1 (1) | <0.001 |
| Physical function measurements | ||||
| 3000-m run (s) | 878 (840–959) | 870 (834–930) | 1027 (985–1084) | <0.001 |
| 2-min push-ups numbers | 45 (40–50) | 45 (40–50) | 25 (21–33) | <0.001 |
| 2-min sit-ups numbers | 45 (40–50) | 45 (40–50) | 35 (30–40) | <0.001 |
| Lung function measurements | ||||
| FEV1 | ||||
| Actual (L) | 3.4±0.6 | 3.5±0.5 | 2.6±0.4 | <0.001 |
| % predicted | 90.8±14.3 | 90.8±14.7 | 90.9±10.9 | 0.926 |
| Z-score | −0.69±1.0 | −0.68±1.0 | −0.74±0.9 | 0.494 |
| FVC | ||||
| Actual (L) | 4.0±0.7 | 4.2±0.6 | 3.0±0.5 | <0.001 |
| % predicted | 93.0±14.3 | 92.9±13.6 | 93.5±11.3 | 0.587 |
| Z-score | −0.55±1.0 | −0.55±1.0 | −0.54±0.9 | 0.844 |
| FEV1/FVC % | ||||
| Actual (%) | 84.4±9.3 | 84.3±9.0 | 85.3±11.9 | 0.205 |
| % predicted | 86.8±1.9 | 86.7±2.0 | 86.9±1.5 | 0.254 |
| Z-score | −0.25±1.2 | −0.23±1.2 | −0.38±1.1 | 0.171 |
FEV1, forced expiratory volume in 1s; FVC, forced vital capacity.
Data are presented as number (percent) for smoking, alcohol, and betel nut. Physical function measurements were presented as median (interquartile range). For all other variables, means±SD are reported.
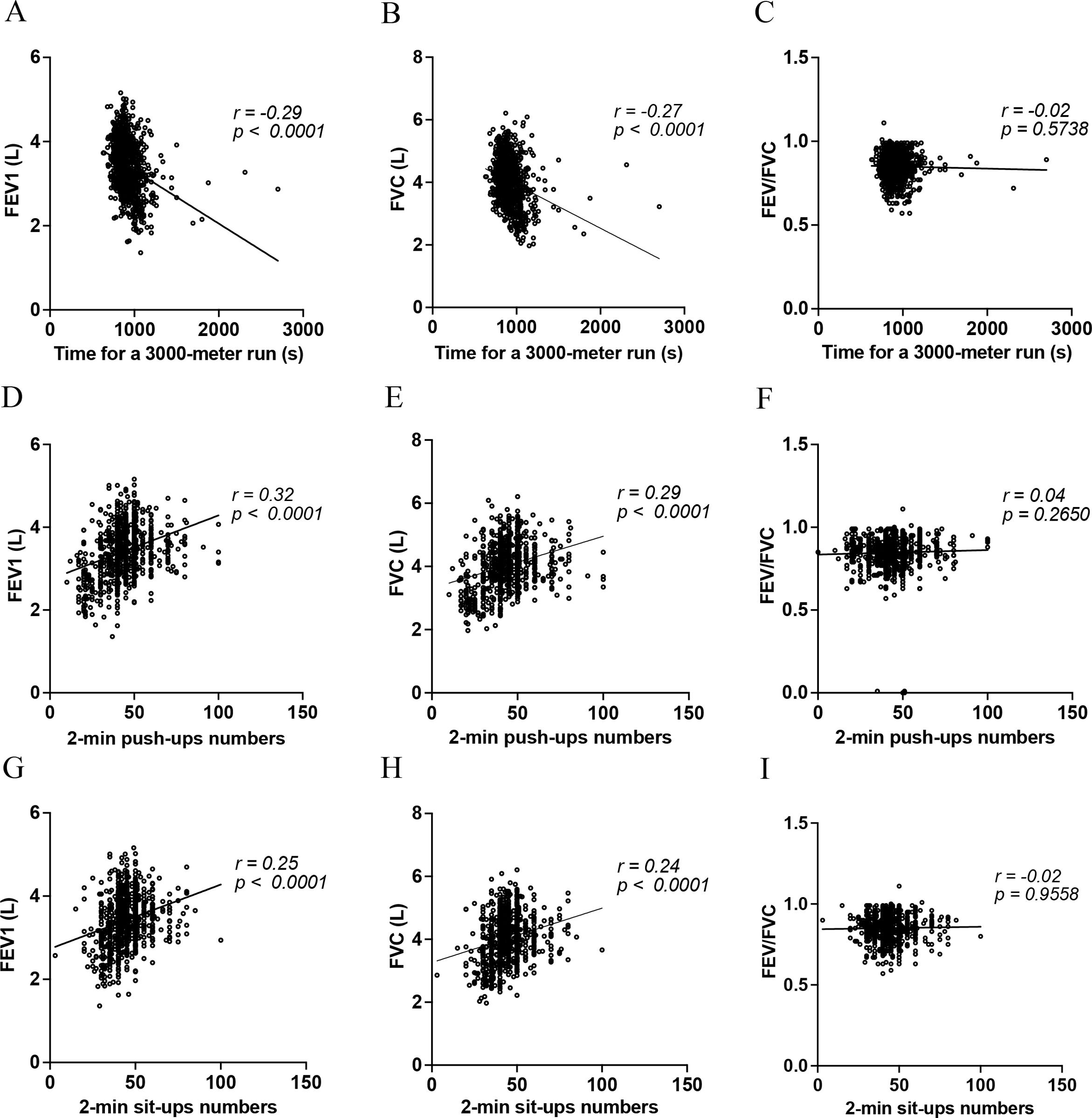
Pearson's correlation coefficients were used to assess the relationship between spirometer parameters and physical performance tests, specifically focusing on CRF and MEF. The relationship between pulmonary function and performance in the 3000-m run test, as well as the outcomes of the 2-minute push-up and sit-up tests, is illustrated in Fig. 2. There is a negative correlation between the time for a 3000-m run and pulmonary function, with FEV1 (Fig. 2A) and FVC (Fig. 2B) both decreasing as the run time increases (r=−0.29, p<0.0001 for FEV1; r=−0.27, p<0.0001 for FVC). No significant correlation is observed with the FEV1/FVC ratio. Furthermore, there is a positive correlation between the number of 2-min push-ups and pulmonary function, with both FEV1 (Fig. 2C) and FVC (Fig. 2D) increasing as the number of push-ups increases (r=0.32, p<0.0001 for FEV1; r=0.29, p<0.0001 for FVC). Again, no significant correlation is observed with the FEV1/FVC ratio. Similarly, a positive correlation is shown between the number of 2-minute sit-ups and pulmonary function, with FEV1 (Fig. 2E) and FVC (Fig. 2F) both increasing with a greater number of sit-ups performed (r=0.25, p<0.0001 for FEV1; r=0.24, p<0.0001 for FVC). The FEV1/FVC ratio does not show a significant correlation. These results suggest that better performance in fitness tests, specifically faster run time and higher numbers of push-ups and sit-ups, is associated with better pulmonary function, as measured by FEV1 and FVC. However, these fitness performances do not appear to affect the FEV1/FVC ratio significantly. We also analyzed the correlation between physical fitness and standardized lung function using Z-scores. The results showed that when lung function was presented as Z-scores, there was no significant correlation between physical fitness and lung function (Fig. E1, Supplementary material). To ensure the results were not influenced by outlier effects, we excluded outliers defined by more than 3 standard deviations from the mean for physical fitness and lung function. Despite this, the correlation between absolute lung function measurements and physical fitness remains significant (Fig. E2, Supplementary material). Given the established correlation between tobacco consumption and physical capacity, we stratified physical fitness by cigarette smoking status. The results show that active smokers exhibited better physical fitness performance in activities such as the 3000-m run, 2-min push-ups, and 2-min sit-ups (Fig. E3, Supplementary material).
Scatter plots representing relationships between lung function metrics and physical tests: (A–C) Correlations of FEV1, FVC, and FEV1/FVC with 3000-m run time. (D–F) FEV1, FVC, and FEV1/FVC with 2-min push-up count. (G–I) FEV1 and FVC with 2-min sit-ups. The correlation coefficient (r) and p-value for each relationship are indicated above each plot. FEV1, forced expiratory volume in 1s; FVC, forced vital capacity; s, seconds. Time is represented in seconds (s).
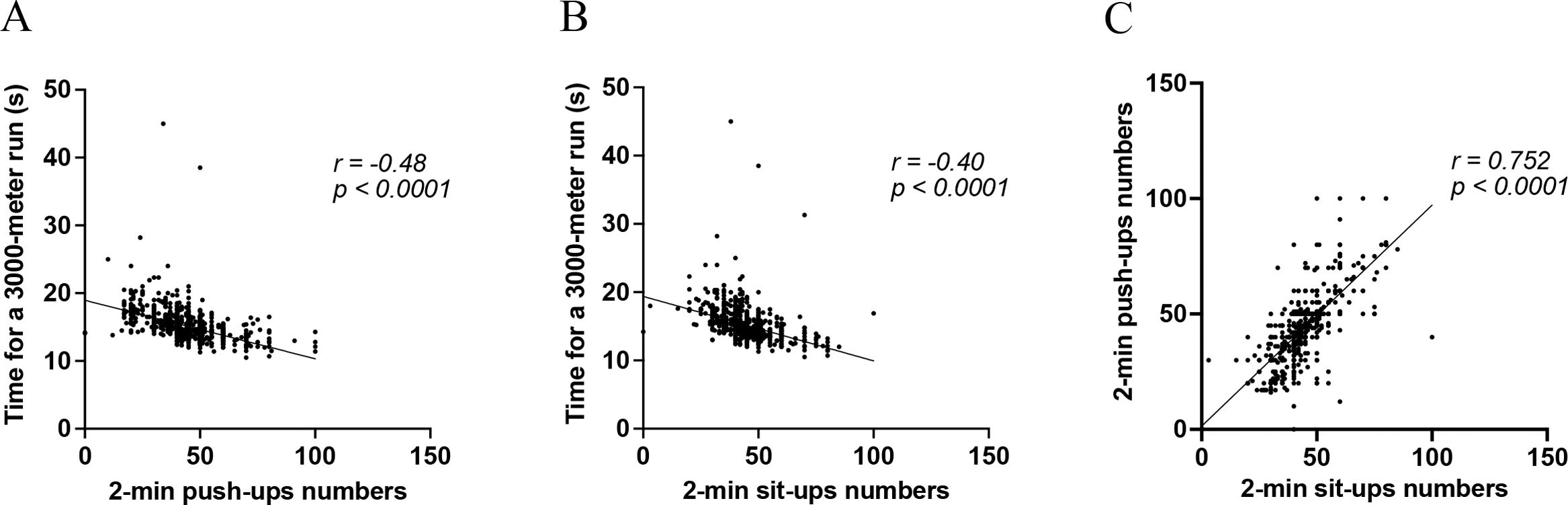
To elucidate the relationship between CRF and MEF, data from scatter plots depicted in Fig. 3A reveal a notable negative correlation (r=−0.48, p<0.0001) between the number of 2-min push-ups and time for a 3000-m run. Higher numbers of push-ups correlate with shorter run times, suggesting enhanced CRF. Similarly, a negative correlation (r=−0.40, p<0.0001) between 2-minute sit-up numbers and run time is shown in Fig. 3B, indicating that greater muscular endurance fitness from sit-ups is associated with improved CRF. The relationship is further underscored in Fig. 3C, which shows a strong positive correlation (r=0.752, p<0.0001) between performance in push-ups and sit-ups, indicating a consistent link between these two forms of exercise. Collectively, these findings highlight the interrelation of MEF and CRF, as well as the association between endurance in upper and lower limb exercises.
Scatter plots illustrating relationships: (A) 3000-m run time vs. 2-min push-ups, (B) 3000-m run time vs. 2-min sit-ups, and (C) 2-min push-ups vs. 2-min sit-ups. The correlation coefficient (r) and p-value for each relationship are indicated above each plot. Time is represented in seconds (s).
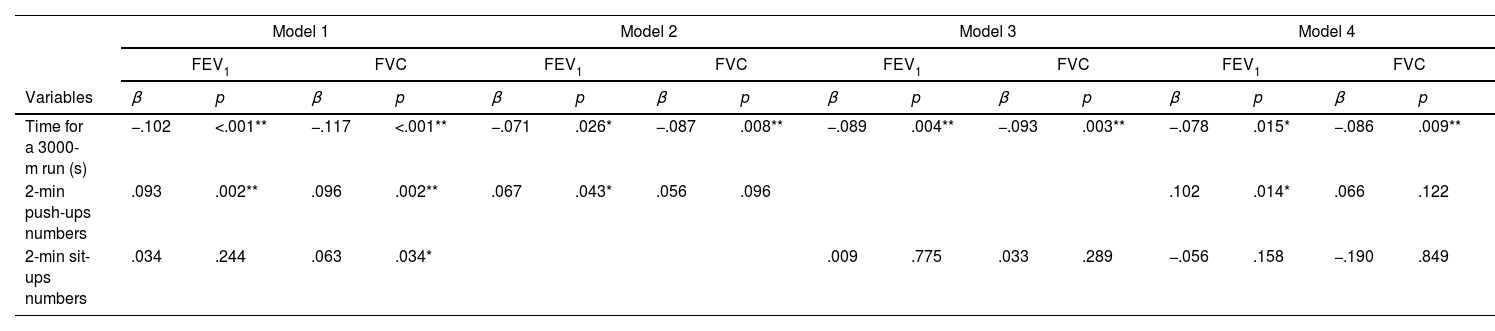
To determine the distinct impacts of various physical fitness modalities on pulmonary function, we utilized Multivariable linear regression analyses, as depicted in Table 2. In Model 1, after adjusting for variables such as age, gender, height, weight, WC, MAP, and smoking status, the results showed that time for a 3000-m run was inversely associated with both FEV1 (standardized β: −.102, p<.001) and FVC (standardized β: −.117, p<.001). Additionally, 2-min push-ups positively correlated with both FEV1 (standardized β: .093, p=.002) and FVC (standardized β: .096, p=.002). In contrast, 2-min sit-ups were only positively associated with FVC (standardized β: .063, p=.034) and not with FEV1.
Multivariable linear regression analysis for the correlations of various physical fitness modalities with pulmonary functions.
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FEV1 | FVC | FEV1 | FVC | FEV1 | FVC | FEV1 | FVC | |||||||||
| Variables | β | p | β | p | β | p | β | p | β | p | β | p | β | p | β | p |
| Time for a 3000-m run (s) | −.102 | <.001** | −.117 | <.001** | −.071 | .026* | −.087 | .008** | −.089 | .004** | −.093 | .003** | −.078 | .015* | −.086 | .009** |
| 2-min push-ups numbers | .093 | .002** | .096 | .002** | .067 | .043* | .056 | .096 | .102 | .014* | .066 | .122 | ||||
| 2-min sit-ups numbers | .034 | .244 | .063 | .034* | .009 | .775 | .033 | .289 | −.056 | .158 | −.190 | .849 | ||||
Multivariable linear regression analysis was used to determine the associations between physical performance and lung functions, with the following adjustments, including exposures (different types of physical performance) and confounders. Model 1: age, gender, height, weight, waist, mean blood pressure, smoking; Model 2: age, gender, height, weight, waist, mean blood pressure, smoking, 3000m-run time, and 2-min push-up numbers; Model 3: age, gender, height, weight, waist, mean blood pressure, smoking, 3000m-run time, and 2-min sit-up numbers; Model 4: age, gender, height, weight, waist, mean blood pressure, smoking, 3000m-run time, and 2-min push-up numbers, 2-min sit-up numbers; β denotes standardized β coefficient.
To explore the potential associations between these physical activities and lung function, we formulated Model 2–4. These models incorporated the same variables as Model 1, with the addition of either a 3000-m run, 2-min push-ups, or 2-min sit-ups. In both Model 2 and Model 3, the duration of the 3000-m run consistently demonstrated an inverse correlation with FEV1 (standardized β: −.071, p=.026; standardized β: −.089, p=.004) and FVC (standardized β: −.087, p=.008; standardized β: −.093, p=.003). Nevertheless, 2-min push-ups only positively correlated with FEV1 (standardized β: .067, p=.043) but not with FVC after adjusting for time for the 3000-m run. After making the same adjustment, the relationship between 2-min sit-ups and lung functions became non-significant.
After adjusting for all covariates and incorporating the three physical activities into the Model 4, time for the 3000-m run continued to show a negative relationship with FEV1 (standardized β: −.078, p=.015) and FVC (standardized β: −.086, p=.009). Notably, the number of 2-min push-ups maintained its positive relationship with FEV1 (standardized β: .102, p=.014) even after adjusting for time for the 3000-m run and 2-min sit-ups, indicating its unique and independent association. In contrast, when considering the performances of time for the 3000-m run and 2-min push-ups, the number of 2-min sit-ups lacked a significant relationship with both FEV1 and FVC. In addition, we also calculated a p-interaction value in Table E1 (Supplementary material) to determine whether sex moderates the association between physical fitness and lung function. The results showed that sex has no interaction effect on the association between physical fitness and lung function.
To investigate the contribution of lung function to maximum physical fitness, we conducted a multivariate linear regression analysis, adjusting for covariates such as age, gender, height, weight, MAP, and smoking status. The analysis revealed that pulmonary function measures, when expressed as Z-scores, did not show significant correlations with physical fitness tests (Table E2, Supplementary material). In contrast, the absolute values of FEV1 and FVC were positively correlated with enhanced performance in the 3000-m run and push-ups (Table E3, Supplementary material). Additionally, FVC, but not FEV1, was associated with improved sit-up performance.
DiscussionIn this cross-sectional study of 1227 active young adults, we explored how physical fitness levels affect lung function. Results showed that better performance in CRF, via 3000-m run times, and MEF, assessed through 2-min push-ups and sit-ups, correlated positively with FEV1 and FVC, but not with the FEV1/FVC ratio. We found a moderate link between 3000-m run times and push-up/sit-up numbers, with a strong association between push-ups and sit-ups. Multivariable linear analysis revealed that shorter run times were correlated with higher FEV1 and FVC, independent of MEF outcomes. Notably, push-ups positively influenced FEV1 alone, while sit-ups showed no significant lung function effect in the fully adjusted model.
Our study found that improved CRF positively correlates with FEV1 and FVC in active young adults, consistent with other research across various demographics.9,19–22 A longitudinal study tracking children into adulthood affirmed this positive CRF association with FEV1 and FVC throughout all ages, but not with the FEV1/FVC ratio.9 Enhancements in CRF during youth were linked to improved FEV1 and FVC in later life. This beneficial association between CRF and ventilatory lung function has been observed in diverse populations, including middle-aged,21 and elderly cohorts,19 and the general population.20,22 Importantly, CRF might not just enhance lung volume but could also improve lung diffusing capacity (DLCO).19,21 These consistent findings across studies and among different age groups highlight the substantial and positive impact of CRF on lung function throughout the life span.
The gold standard for assessing CRF is Cardiopulmonary Exercise Testing (CPET), which evaluates VO2max or VO2peak, and can be estimated from work rates or algorithms.23 While previous research has linked CRF to lung function via VO2max9 or VO2peak19–22 measurements through CPET, its requirement for specific facilities and participant mask tolerance during testing limits its practicality, especially for children, the elderly, or large-scale studies.24 The American Heart Association (AHA) endorses CPET and run field tests for assessing VO2max in youths.25 Our prior research has found a significant correlation (r=0.729, p<0.001) between 3000-m run times and VO2max scaled to body mass in young adults, indicating that the 3000-m run is an effective, accessible method for estimating CRF, suitable for larger populations or settings with limited resources.17
The correlation between decreased pulmonary function and impaired peripheral muscle strength and endurance has been established in patients with chronic obstructive pulmonary disease (COPD).26–30 A case–control study showed that handgrip muscle strength decreases as the FEV1 and FVC decrease in patients with COPD compared to the normal subjects.29 Moreover, it was observed that COPD patients experienced a pronounced decline in muscle endurance compared to muscle strength. Several studies have also confirmed a positive correlation between handgrip muscle strength and lung function across diverse populations, ranging from adolescents to the elderly.15,24,31–36 These results suggest that an association between muscular strength and lung function was observed not only in COPD patients but also in the general community population. Nevertheless, the impact of various forms of peripheral muscle endurance on lung function has been poorly investigated in previous studies, yielding inconsistent results. One study found a positive association between muscle endurance, assessed by quadriceps, and FEV1 in COPD patients,26 but another study failed to identify a similar association when evaluating muscle endurance through handgrip.29 These discrepancies might arise from the use of various muscle groups and measurement techniques, variability in the study populations, and small sample sizes. Our study examined the relationship between both upper and lower limb muscle endurance, separately measured by the number of 2-min push-ups and sit-ups, and lung function, revealing a positive correlation in a large cohort of physically active young adults without chronic lung diseases. Initially, both 2-min push-ups and sit-ups showed a positive correlation with FEV1 and FVC in univariate analysis. Nevertheless, the link between 2-minute sit-ups and FEV1 became insignificant after adjusting for covariates. In contrast, the 2-min push-ups maintained a positive correlation with FEV1, even after accounting for additional physical fitness metrics such as 2-min sit-ups and 3000-m run time. These findings indicate that upper limb muscle endurance has a greater impact on lung function, particularly on FEV1, compared to lower limb muscle endurance. Consequently, our findings propose that the 2-min push-ups fitness routine, focused on improving upper limb muscle and pectoral muscle endurance, might be beneficial in managing obstructive lung diseases like COPD or asthma with airflow limitation. Particularly, the performance of the 3000-m run remained positively correlated with both FEV1 and FVC across all models, indicating its robust impact on overall lung function.
The mechanisms connecting improved or enhanced physical fitness with better lung function are not fully understood. It is plausible that regular exercise training, a determinant of CRF, may strengthen the respiratory muscles, enhance pulmonary perfusion as well as surfactant release, and ultimately lead to increased lung volumes.37–39 Additionally, enhanced fitness may decrease fat distribution, particularly abdominal and thoracic fat mass, which can constrain vital capacity and lead to expiratory flow limitation by restricting lung expansion during inhalation.40 Moreover, reduced fat mass may also alleviate lung function decline by reducing inflammatory processes, as adipose tissue is a source of inflammatory mediators that can harm lung tissue and narrow airway diameter.41 The positive correlation between the enhanced 2-min push-up test and lung function might be attributed to the fact that push-ups activate not only the arms and chest but also the accessory respiratory muscles, including the intercostal muscles, pectoral muscles, and those around the shoulder girdle. Furthermore, the repetitive and sustained strengthening of these muscles could improve their efficiency and capacity, thereby contributing to improved lung function.
In the study, we analyzed the correlation between lung function and physical fitness, presenting lung function as either absolute values or Z-scores, which resulted in inconsistent findings. The inconsistency in the correlation results between absolute lung function values and physical fitness versus standardized lung function (Z-scores) could be partly explained by the attenuation of the association when anthropometric variables were accounted for at an individual level. When lung function is presented with absolute lung volumes, the measurements reflect the remaining ventilatory capacity, which may correlate more directly with physical fitness due to the physical demands on lung capacity during exercise.42 However, when lung function is standardized into Z-scores, these values are adjusted based on a population mean and standard deviation, accounting for variations due to factors like age, sex, and height.1 This standardization can obscure the raw differences seen with absolute values. For example, in a cross-sectional study, males and females, even when matched on relative lung volume (like FEV1% predicted), had significantly different absolute lung volumes, with males having a higher volume.43 This discrepancy can alter perceptions of lung capacity and its relation to physical fitness when using standardized scores. Therefore, the inconsistency in the results could arise from these underlying differences in measurement and interpretation, highlighting the need for future studies to determine which lung function measurement is appropriate to correlate with physical fitness.
Several strengths and limitations of our study warrant discussion. This research is significant for being the first to investigate the relationship between different physical fitness aspects, including CRF and MEF, and their specific impacts on lung function in a cohort of physically active young adults. It provides valuable benchmarks for physical performance and lung function metrics within this group, known for their peak physiological capacities, including optimal lung and cardiovascular efficiency. Our research uniquely focuses on active young adults without chronic lung conditions, offering clearer insights into the fitness and lung function link. However, the cross-sectional, observational design limits our ability to assert causality. Although enhanced lung function may be attributed to factors common to both exercise capacity and pulmonary metrics, the FEV1 measurement remains positively correlated in the fully adjusted model, underscoring a distinct connection between push-ups and FEV1. Furthermore, our results indicate that active smokers demonstrated superior physical fitness performance in activities such as the 3000-m run, 2-min push-ups, and 2-min sit-ups. Nevertheless, it is important to note that our participant group is predominantly young, and detailed quantitative data on cigarette consumption and smoking duration are not available. Consequently, we are unable to conclusively determine the effects of smoking on physical performance. This limitation underscores the need for further research with more comprehensive smoking data to robustly assess the impact of tobacco use on physical fitness. Finally, the participant demographic, primarily Asian males, may not reflect the diversity needed for broader applicability. These points highlight the necessity for future studies with a prospective design, encompassing a wide range of ethnic backgrounds and a balanced gender distribution, to comprehensively understand the causal relationship between MEF and pulmonary function.
In conclusion, this study demonstrated that improved CRF and MEF are positively associated with pulmonary function in active young adults. It notably suggests that push-ups are distinctly associated with increased FEV1, highlighting the potential of specific physical activities to influence lung health. Further research is required to determine whether routine exercises like push-ups can consistently enhance lung function among various demographic and health backgrounds.
Sources of fundingThis research was funded by TSGH, through awards TSGH-D-113114, and supported by the Medical Affairs Bureau of the Ministry of National Defense, under awards MND-MAB-D-113200.
Author's contributionsG.M.L. designed the study. C.H.L. performed the data analysis. C.H.L. and G.M.L. interpreted the results of statistical analysis. C.H.L. wrote the manuscript. G.M.L., Y.C.L., W.C.H., X.S., and C.J.L. revised the manuscript critically for important intellectual contents. All authors contributed to the interpretations of the findings. All authors reviewed the manuscript.
Role of the sponsorsThe sponsors and funders of the study had no involvement or any influence in the study design, in the collection, analysis, and interpretation of data, in the writing of the manuscript, and in the decision to submit the manuscript for publication. The corresponding author confirms that he had full access to all the data and had the final responsibility for the decision to submit for publication.
Data sharing statementThe datasets supporting the conclusions of this article will be made available by the authors upon reasonable request, subject to necessary approvals and confidentiality agreements. Specific data that are not publicly available due to privacy or ethical restrictions will be accessible subject to conditions outlined by the corresponding author.
Conflict of interestNo conflicts exist for the authors.
We extend our deepest gratitude to all participants and collaborators who made this study possible. Special thanks to TSGH and Ministry of National Defense R.O.C. for their financial support.