Introduction
Current treatment for chronic obstructive pulmonary disease (COPD) is aimed at increasing airflow, decreasing respiratory symptoms (particularly dyspnea), decreasing exacerbations, and improving the quality of life. Consequently, it not surprising that both the Global Initiative for Obstructive Lung Disease (GOLD) guidelines1, and the recent American Thoracic Society (ATS)/European Respiratory Society (ERS) position paper for the diagnosis and treatment of patients with COPD2 emphasize the role of bronchodilators in symptomatic management of all stages of COPD.
There are two primary pharmacologic classes of inhaled bronchodilators with different mechanisms of action which are used in the treatment of COPD: β2-adrenoceptor (β2-AR) agonists and quarternary ammonium anticholinergic agents3, which can be either short-acting (e.g., salbutamol, terbutaline, ipratropium or oxitropium) or long-acting (e.g., formoterol, salmeterol, or tiotropium). Both the GOLD Update and the ATS/ERS position paper recommend, for moderate-to-very severe COPD, use of regular treatment with long-acting bronchodilators rather than short acting bronchodilators, with the choice depending on the availability of medication and the patient's response1,2.
For patients whose conditions are not sufficiently controlled by monotherapy, these guidelines highlight that combining medications of different classes, in particular an inhaled anticholinergic with a β2-AR agonist, seems a convenient way of delivering treatment and obtaining better results1,2. This includes better lung function and improved symptoms.
Rationale for combining β2-adrenoceptor agonists and anticholinergic agents
Postganglionic, parasympathetic-cholinergic nerves innervate the airways. When activated, these nerves are capable of obliterating the lumen of small bronchi and bronchioles, and markedly increasing airway resistance in larger, cartilaginous airways, by secretion of the bronchoconstricting mediator acetylcholine (ACh), which causes activation of muscarinic receptors at the level of the target cells, such as bronchial smooth muscle and goblet cells4. Conversely, sympathetic nerves may control tracheobronchial blood vessels, but no innervation of human airway smooth muscle has been demonstrated. β2-ARs, however, are abundantly expressed on human airway smooth muscle and activation of these receptors causes its relaxation5.
Bronchodilatation may, therefore, be obtained either by stimulating the β2-ARs with β2-AR agonists, or by inhibiting the action of ACh at muscarinic receptors with anticholinergic agents. In any case, anticholinergics are more likely to decrease central airway resistance, although there are muscarinic receptors that are expressed in the smooth muscle of small airways which do not appear to be innervated by cholinergic nerves, and β2-AR agonists have a greater effect on peripheral airway resistance in patients with COPD6. It is reasonable to postulate that attempts to reduce bronchoconstriction through two distinct mechanisms (anticholinergic and sympathomimetic) with a different prevalent site of action may maximize bronchodilator response.
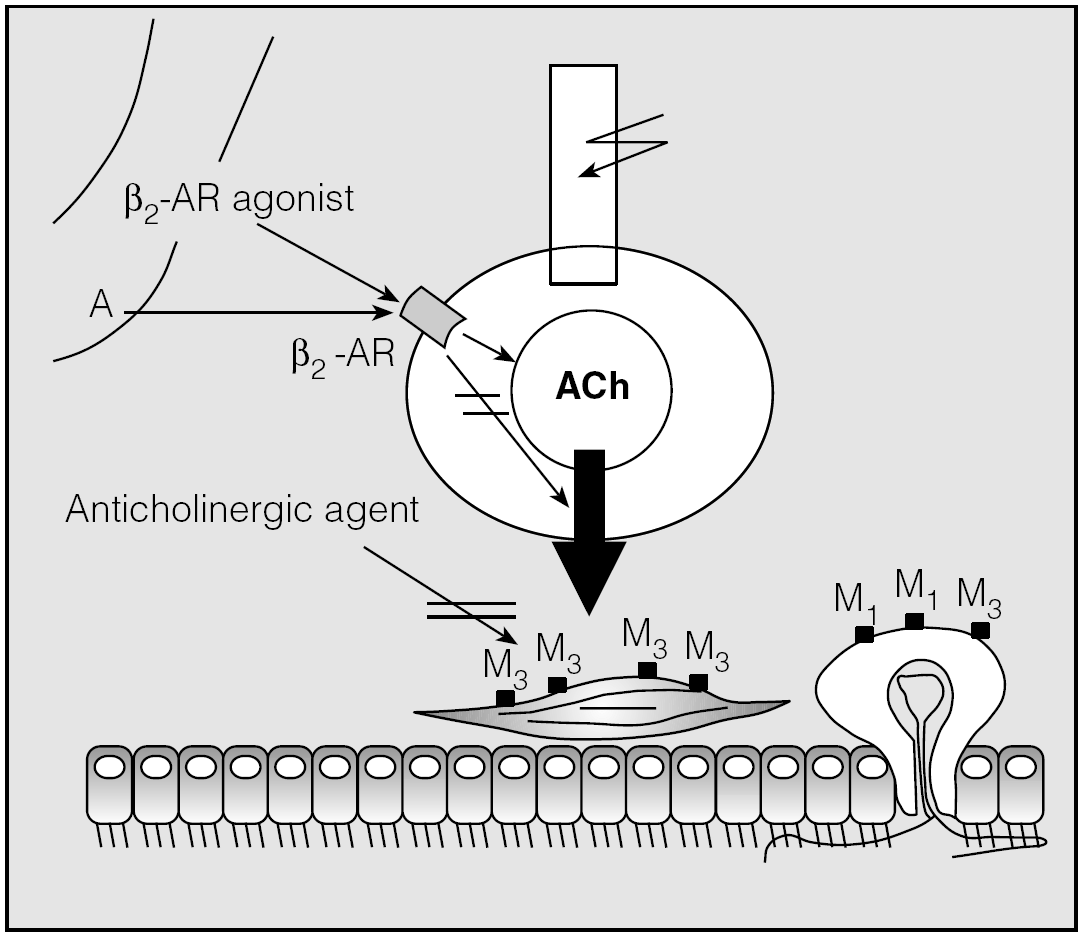
Interestingly, the presence of small dense-cored vesicles containing adrenergic nerve varicosities, occasionally in close proximity to morphologically characteristic cholinergic nerve-endings, has been identified in human airways7, suggesting that catecholamines might modulate cholinergic neurotransmission (fig. 1). However, different studies have led to dissimilar conclusions. Early research papers based on force measurement alone suggested that stimulation of β2-ARs inhibits cholinergic neurotransmission8, most probably by the release of inhibitory prostaglandins from the airway mucosa9. However, interpretation of these data is seriously hampered by the large postjunctional effects of β2-AR agonists. Zhang et al10 were the first to report an excitatory β2-AR in airway parasympathetic nerves. After their report in studies on the horse, this receptor was subsequently reported in guinea pigs11 and in human airway parasympathetic nerves12. Activation of β2-ARs by isoproterenol, by the racemic mixture of specific β2-AR agonists such as salbutamol and formoterol or by R-enantiomers of β2-agonists can increase ACh release in a concentration-dependent manner13. Consistent with the results of β2-AR stimulation14, direct activation of the β2-AR-coupled Gs protein by cholera toxin, which increases the activity of adenylyl cyclase, caused an increase in ACh release in epithelium-denuded guinea pig trachealis11. In airway smooth muscle cells, however, stimulation of Gs protein directly opens large Ca2+-activated potassium (KCa) channels15, which has been found to decrease ACh release in guinea pig trachealis16. Recently, Brichetto et al17 have confirmed that β2-AR agonists attenuate cholinergic neurotransmission in the isolated bovine trachealis model and this happens by a mechanism not involving cAMP but KCa channels.
Fig. 1. Schematic presentation of the potential alternative role of β2-adrenoceptor (AR) in the pre-synaptic control of acetylcholine (ACh) release from airway parasympathetic nerve endings. A: circulating adrenaline; β2-AR: β2-adrenoceptor; M1 and M3: muscarinic M1 or M3 receptors; (>: neuronal activity; →: stimulatory effect; (=↓: inhibitory effect.
Whatever the type of interaction between the two systems may be, combining β2-AR agonists and anticholinergic agents is pharmacologically useful. In fact, in the first case, the addition of a β2-AR agonist decreases the release of ACh because of the modulation of cholinergic neurotransmission by prejunctional β2-ARs and, consequently, amplifies the bronchial smooth muscle relaxation directly induced by the anticholinergic agent. On the contrary, in the second circumstance, the addition of an anticholinergic agent can reduce the peripheral bronchoconstrictor effects of ACh, whose release has been facilitated by the β2-AR agonist, and in this manner can amplify the bronchodilation elicited by the β2-AR agonist through the direct stimulation of smooth muscle β2-ARs.
Combination therapy with ipratropium and a β2-agonist
It has been documented that standard doses of short acting β2-agonists do not give optimal results in patients with COPD and that an anticholinergic agent gives additional bronchodilation18. In addition, the reproducibility of responsiveness to bronchodilators in patients with COPD is improved when the pulmonary function test is performed using a combination of ipratropium and salbutamol18. In effect, although a few articles question the value of combination therapy with ipratropium bromide and a β2-AR agonist19,20, several large trials suggest that the two drugs, both at low doses21 and at high doses22,23, have complementary, or additive, bronchodilator actions without any increase in the incidence of adverse reactions, which makes them an excellent combination for the treatment of COPD. The inclusion of a second agent of a different class in a pharmacologic treatment regimen is associated with a lower rate of exacerbations in COPD24. The overall result is lower total treatment costs and improved cost-effectiveness.
The introduction of long acting β2-AR agonist bronchodilators gives physicians additional therapeutic options for COPD. Both salmeterol and formoterol appear to be more effective than short acting β2-AR agonists25 and in patients with stable COPD they are more effective than anticholinergic agents26-28.
Two published studies that had evaluated small cohorts suggested that there is no substantial additive effect when a long-acting β2-AR agonist is combined with ipratropium bromide given acutely at the clinically recommended dose (40 μg) in patients with COPD29,30, but it must be stressed that the dose of ipratropium bromide needed to produce near maximal bronchodilation is several times higher than the customary dosage31. In fact, the results of some studies suggest that higher than normal doses of an anticholinergic drug must be used for further relief of bronchospasm in patients with COPD when a single conventional inhaled dose of formoterol32 or salmeterol33 is given first.
In any case, van Noord et al34 demonstrated that a 12-week treatment with salmeterol 50 μg twice daily plus ipratropium bromide 40 μg four times daily was more effective than salmeterol 50 μg twice daily in improving forced expiratory volume in 1 second (FEV1) and specific airway conductance. This study was the first to point out that the association of a long acting β2-AR agonist and an anticholinergic agent is useful in the long term therapy of stable COPD.
Subsequently, D'Urzo et al35 not only confirmed this therapeutic possibility, but even documented that the addition of formoterol (12 μg twice daily) to ipratropium bromide (40 μg four times a day) is more effective than the addition of salbutamol (200 μg four times a day) in patients with COPD who required combined bronchodilator therapy. This finding clearly indicates that long-acting β2-AR agonists may represent the most effective option for combination therapy with an antimuscarinic agent.
The functional impact of combining long-acting bronchodilators
Clinical studies show that tiotropium administered 18 μg once daily improves lung function over its 24-h dosing interval, as shown by FEV1, forced vital capacity (FVC), peak expiratory flow rate (PEFR) and measures of hyperinflation and provides superior spirometric improvements compared with ipratropium 40 μg four times daily36. Considering this important finding, in an elegant review in which the pharmacological actions of the long-acting β2-AR agonists and a long-acting muscarinic antagonist (tiotropium bromide) were summarized, Tennant et al37 highlighted the need for investigating the combination of tiotropium bromide with a long-acting β2-AR agonist. Afterwards, Tashkin and Cooper38, using the traditional method of integrating research studies that, unfortunately, does not allow to determine if the differences between the study outcomes are due to chance, to inadequate study methods or to systematic differences in the characteristics of the studies, emphasized the advantage of tiotropium on long-acting β2-agonists, and suggested adding salmeterol or formoterol to tiotropium bromide, at least in patients suffering from COPD with more severe symptoms (stage III or IV of the GOLD classification1. However, Cazzola and Matera39 in an accompanying editorial comment highlighted that no published study has documented the superiority of tiotropium over formoterol, although two studies, specifically designed to explore the potential differences between tiotropium and salmeterol, seem to indicate a greater efficacy of tiotropium than long-acting β2-AR agonists40,41. The different pharmacodynamic profile of formoterol when compared to salmeterol25 might induce a different type of broncholytic effect, mainly if one considers onset of action or peak bronchodilation. This may lead to a different conclusion when comparing formoterol with salmeterol. Moreover, it is not known if the combination of a long-acting β2-AR agonist and a long-acting antimuscarinic agent provides further advantages in terms of bronchodilation over either drug alone, nor if the choice of the specific long-acting β2-agonist to be used is trivial.
Acute functional effect of combining formoterol or salmeterol and tiotropium
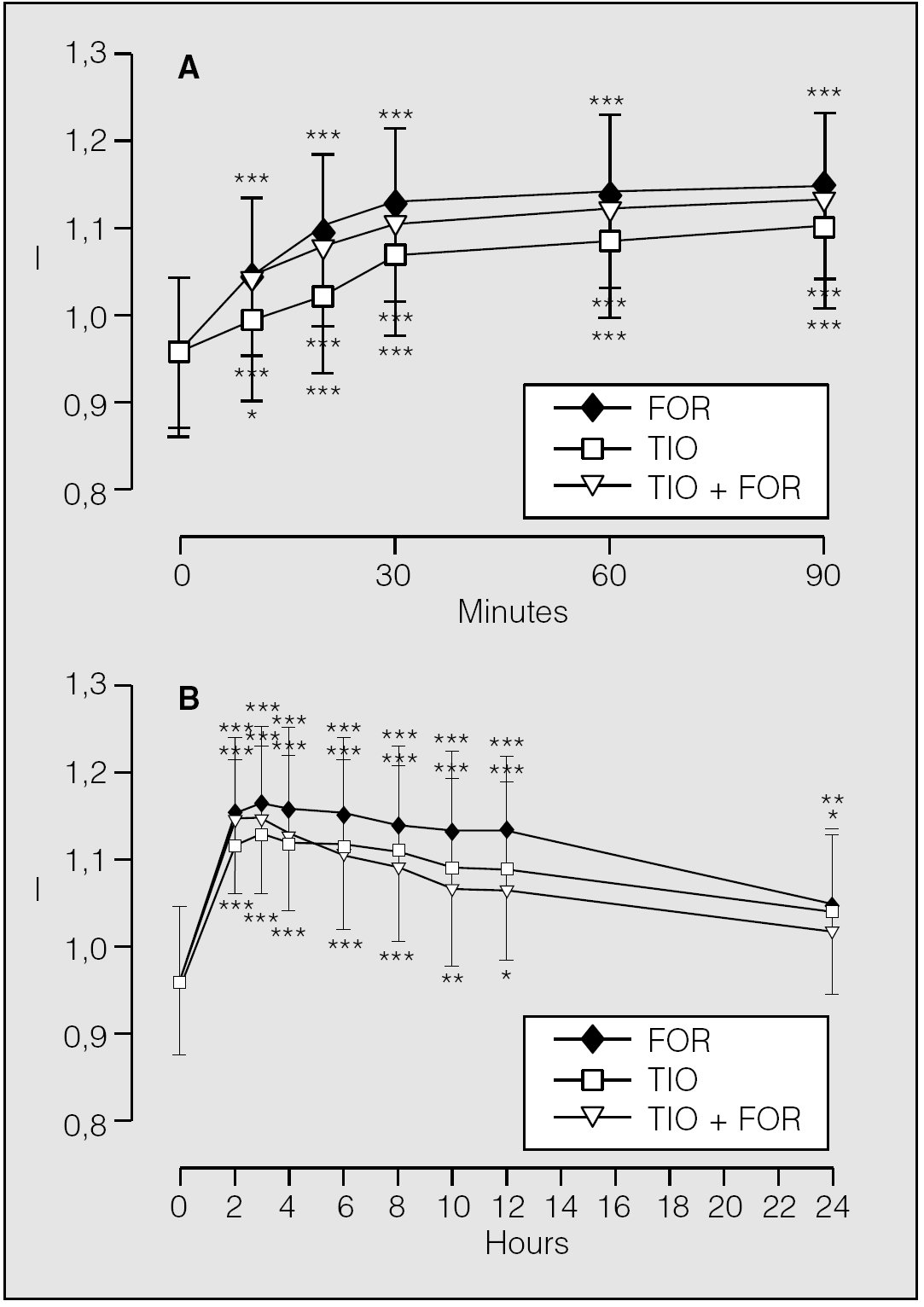
Some studies have tried to give an answer to these questions. A pilot investigational trial42, which enrolled 20 outpatients clinically diagnosed with stable COPD and a mean baseline FEV1 of 0.87 l (95% confidence interval [CI], 0.70-1.04) and FVC of 1.49 l (95% CI, 1.30-1.69), showed that 12 μg formoterol, either alone or in combination with 18 μg tiotropium, elicited a significantly faster onset of action (the change in FEV1 10 min after inhalation of formoterol alone [0.088 l; 95% CI, 0.049-0.127] was greater than that induced by tiotropium alone [0.039 l; 95% CI, 0.006-0.071], but not than that elicited by formoterol + tiotropium [0.085 l; 95% CI, 0.044-0.126]) (fig. 2 A). Moreover, this study also documented a trend for a greater maximum bronchodilation with combination than with formoterol or tiotropium alone (the mean maximum increases in FEV1 from pre-dosing value on each of the dosing days were 0.192 l [95% CI, 0.125-0.259] for formoterol, 0.176 l [95% CI, 0.100-0.253] for tiotropium, and 0.210 l (95% CI, 0.158-0.261] for the combination and occurred two hours after formoterol and three hours after inhalation of tiotropium and the combination, but the difference between treatments was not significant [p = 0.475]) (fig. 2 B). At twenty four hours, mean FEV1 continued to be significantly higher than pre-dosing value following tiotropium (0.084 l; 95% CI, 0.003-0.134; p = 0.003) and formoterol + tiotropium 0.088 l; 95% CI, 0.002-0.173; p = 0.045), but did not achieve significance for formoterol alone (0.058 l; 95% CI, 0.000-0.117; p = 0.051) (fig. 2 B). However, at this time point, the differences between treatments were not significant (p = 0.731). The failure to show a statistically significant difference between treatments when we explored the maximum bronchodilation and the duration of action was likely associated with an insufficient statistical power in the study. We believe that there was a possibility of a type II error, which supported the lack of significance that we have repeatedly observed. It is possible that a study with a larger sample would achieve statistical significance.
Fig. 2. Mean changes (± SE) in FEV1 (l) from pre-dosing value on each of the dosing days up to 90 min (A) and up to 24 h (B) after inhalation of formoterol 12 μg (FOR), tiotropium 18 μg (TIO), and tiotropium 18 μg + formoterol 12 μg (TIO + FOR). *p < 0.05; **p < 0.01; ***p < 0.001 versus baseline. (Data from Cazzola et al42.)
The results of this study indicate that formoterol and tiotropium have different profiles (formoterol has a faster onset of action and greater bronchodilating effect, tiotropium has a longer duration of action, which allows for once daily administration) that make both agents attractive alternatives in the treatment of stable COPD. Moreover, the two drugs appear complementary: tiotropium ensures prolonged bronchodilation, whereas formoterol adds fast onset and a greater peak effect. However, because formoterol is given twice daily, but tiotropium is required only once daily, and results of our study do not allow suggesting the once daily dosing of formoterol, the challenge is to develop a combined inhaler that can be employed on a daily basis37. The pharmacodynamic characteristics of salmeterol might permit the once daily contemporaneous administration of the two drugs that could simplify the therapy39.
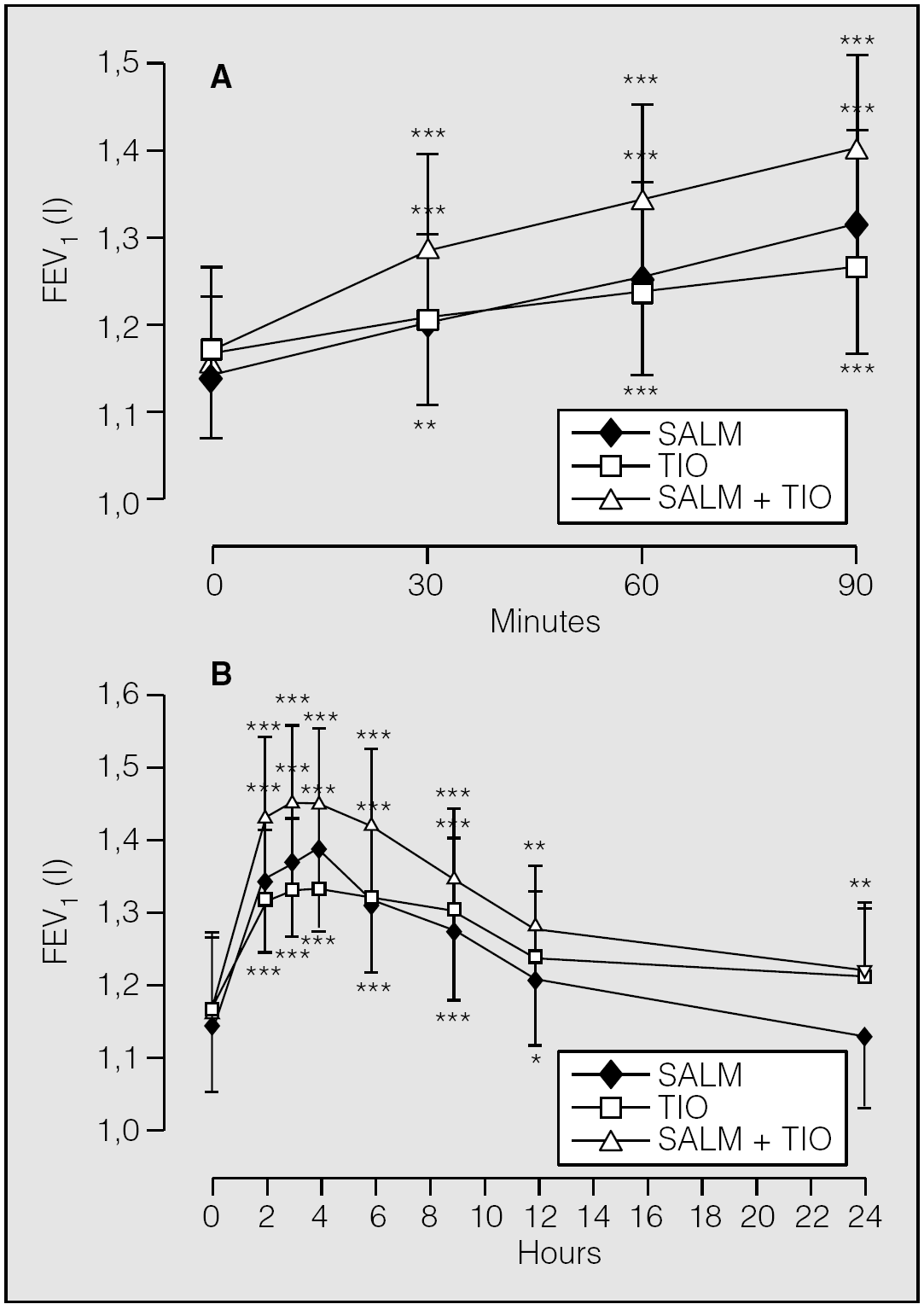
In a further study43 that has enrolled 20 outpatients with stable COPD and a mean baseline FEV1 of 1.10 l (95% CI, 0.91-1.29) and FVC of 1.85 l (95% CI, 1.62-2.07), single doses of 18 μg tiotropium, 50 μg salmeterol, and 18 μg tiotropium + 50-μg salmeterol were given. The change in FEV1 30 min after inhalation of salmeterol alone (0.058 l; 95% CI, 0.032-0.083) was greater than that induced by tiotropium alone (0.037 l; 95% CI, 0.013-0.062), but not than that elicited by tiotropium + salmeterol (0.121 l; 95% CI, 0.073-0.170) (fig. 3 A). The difference between the improvement after salmeterol and that after tiotropium was not statistically significant (p = 0.231), but the differences between the improvement after tiotropium + salmeterol and that after tiotropium alone and salmeterol alone were statistically significant (p = 0.014 and p = 0.026, respectively). The mean maximum increases in FEV1 from pre-dosing value on each of the dosing days were 0.165 l (95% CI, 0.098-0.232) for tiotropium, 0.241 l (95% CI, 0.151-0.332) for salmeterol, and 0.290 l (95% CI, 0.228-0.353) for the combination and occurred four hours after inhalation of tiotropium or salmeterol and three hours after that of the combination (fig. 3 B). At twenty four hours, the mean FEV1 value was still higher than the mean pre-dosing value for tiotropium (0.042 l; 95% CI, 0.012 to 0.097; p = 0.119) and the tiotropium + salmeterol combination (0.051 l; 95% CI, 0.015-0.087; p = 0.008), but not for salmeterol alone (0.013 l; 95% CI, 0.041 to 0.014; p = 0.324) (fig. 3 B). At this time point, the differences between tiotropium and salmeterol and between salmeterol and tiotropium + salmeterol, but not that between tiotropium and tiotropium + salmeterol, were statistically significant (p = 0.010, p = 0.006, and p = 0.752, respectively).
Fig. 3. Mean changes (± SE) in FEV1 (l) from pre-dosing value on each of the dosing days up to 90 min (A) and up to 24 h (B) after inhalation of salmeterol 50 μg (SALM), tiotropium 18 μg (TIO), and salmeterol 50 μg + tiotropium 18 μg (SALM + TIO). *p < 0.05; **p < 0.01; ***p < 0.001 versus baseline. (Data from Cazzola et al43.)
These findings support the possibility of combining tiotropium and salmeterol in patients suffering from stable COPD, but exclude the once-daily co-administration of the two drugs. The potential of salmeterol to increase its onset of action when combined with tiotropium is worthy of attention considering that both agents elicit a slow onset of action44,45. Ethier et al46 documented that at the lowest concentration of salmeterol needed to maximally stimulate cAMP accumulation, this long-acting β2-AR agonist antagonized muscarinic inhibition of cAMP accumulation and altered agonist binding to muscarinic receptors in bovine trachealis cells. Because salmeterol altered agonist, but not antagonist, binding and appeared to have an effect on the proportion of high affinity binding sites, Ethier et al46 speculated that salmeterol has a low affinity binding interaction with the muscarinic receptor/G-protein complex itself or reversibly alters the plasma membrane environment surrounding the complex. In any case, these effects of salmeterol did not depend on stimulation of β2-ARs. If this finding were also true for human airways, we could speculate that salmeterol reduces the bronchospastic activity of endogenous ACh, without influencing the effect of tiotropium. Salmeterol has also high affinity for muscarinic receptors47 and this indicates a potential for amplifying the action of tiotropium. However, the question of whether high local concentrations after inhalation of salmeterol could contribute to its therapeutic effects by antagonism with ACh on muscarinic receptors remains to be answered47.
Functional effect of a regular treatment with formoterol or salmeterol and tiotropium
Although these results indicate that a combination of tiotropium and a long-acting β-AR agonist is more effective than single drugs alone in inducing bronchodilation in patients suffering from COPD, there is a fundamental aspect of these studies, the evaluation of the impact of the three treatments only after acute administration, that must be taken into account. Acute administration is, in fact, a potential bias because it is well known that FEV1 steady state with tiotropium is reached within forty eight hours, while continued improvements in FVC can be expected over or beyond the first week of therapy48. The progressive increases in FVC beyond forty eight hours suggest that maintenance bronchodilator therapy is required to achieve maximal changes in hyperinflation.
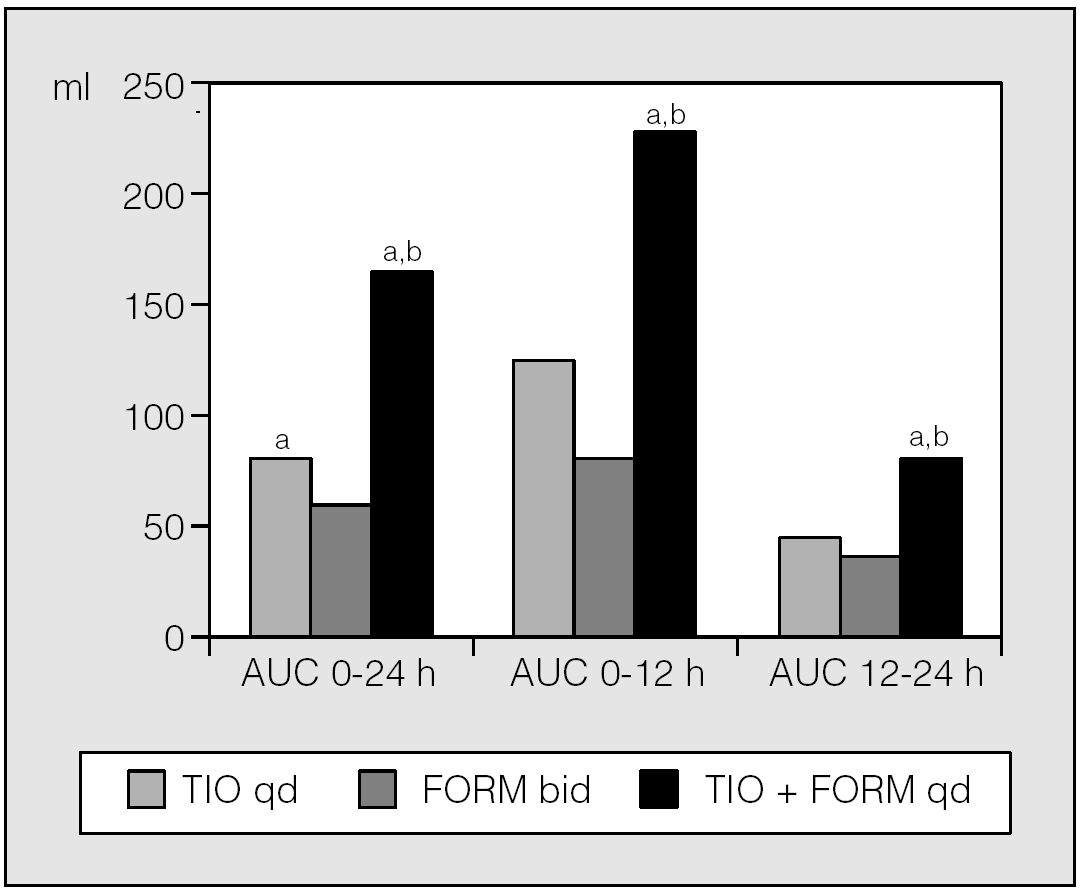
Data of a trial49 that compared the lung function response of the free combination of 18 μg tiotropium plus 12 μg formoterol once daily with 18 μg tiotropium once daily and 12 μg formoterol twice daily, with all treatments administered for 6-week periods, in 66 patients with moderate-to-severe stable COPD, documented that the free combination of once daily tiotropium plus formoterol was better than either of the single drugs for most of the spirometric endpoints. Tiotropium once daily was superior to formoterol twice daily during the daytime. However, during the night-time, tiotropium and formoterol provided similar bronchodilation. In particular, the average FEV1 area under the curve (AUC)0-24h was 85 ml after tiotropium, 62 ml after formoterol and 160 ml after tiotropium + formoterol, the average FEV1 AUC0-12h was 127 ml after tiotropium, 86 ml after formoterol and 234 ml after tiotropium + formoterol, whereas the average FEV1 AUC12-24h was 43 ml after tiotropium, 38 ml after formoterol and 86 ml after tiotropium + formoterol (fig. 4). All FEV1 AUC after tiotropium + formoterol were significantly (p < 0.05) larger than that of tiotropium or formoterol alone. The daytime, but not the night-time, use of rescue salbutamol was significantly reduced in patients receiving the combination of tiotropium + formoterol (p < 0.05 versus either drug alone). The documentation that tiotropium was more active than formoterol in daytime but not in night-time was, in our opinion, in agreement with the fact that the activity in the sympathetic system appears to be prominent during the day as reflected by the peak located around noon of the urinary catecholamine excretion, whereas the vagal system appears to be prominent during the remainder of the day50. In any case, these results indicate that once daily combination therapy of tiotropium + formoterol is safe and provides significant additive effects in patients with moderate-to-severe COPD. Moreover, they suggest that once daily administration of the two drugs could be a possibility in the treatment of stable COPD. A bronchodilator-mediated symptom benefit of the once daily combination is also reflected in significant decrease in salbutamol use as rescue therapy.
Fig. 4. Tiotropium 18 μg once daily (TIO qd), tiotropium 18 μg once daily + add-on formoterol 12 μg once daily (TIO + FORM qd) and tiotropium 18 μg once daily + add-on formoterol 12 μg twice daily (TIO + FORM bid) FEV1 AUCs calculated using the trapezoidal rule divided by the corresponding observation time to report in ml units. ap < 0.05 versus formoterol; bp < 0.05 versus tiotropium. (Data from van Noord et al49.)
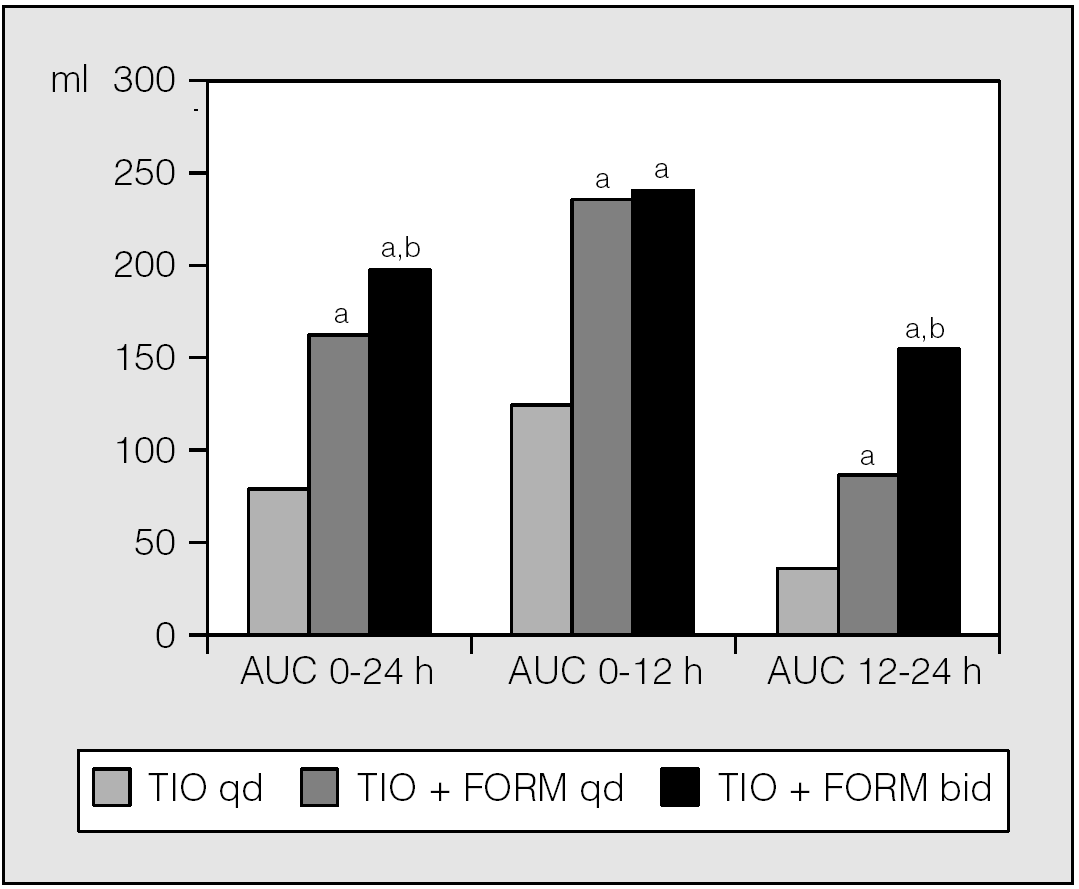
However, another trial that explored tiotropium maintenance therapy in 91 patients with COPD and the twenty four hour spirometric benefit of adding once or twice daily formoterol during two-week treatment periods51, documented that add-on therapy of a second formoterol dose significantly (p < 0.05) improved FEV1 and FVC variables when compared with tiotropium + formoterol once daily, although most of the spirometric add-on benefit was found with the morning formoterol dose. The average FEV1 AUC0-24h was 80 ml after tiotropium, 162 ml after tiotropium + formoterol once daily and 198 ml after tiotropium + formoterol twice daily, the average FEV1 AUC0-12h was 125 ml after tiotropium, 238 ml after tiotropium + formoterol once daily and 241 ml after tiotropium + formoterol twice daily, whereas the average FEV1 AUC12-24h was 37 ml after tiotropium, 89 ml after tiotropium + formoterol once daily and 156 ml after tiotropium + formoterol twice daily (fig. 5). FEV1 AUC12-24h of tiotropium + formoterol twice daily value was significantly (p < 0.05) different from that of tiotropium + formoterol once daily. Inspiratory capacity (IC), which is an index of hyperinflation, showed a circadian rhythm during both the baseline and the treatment periods. The improvements in IC with both tiotropium alone and when combined with formoterol paralleled those in FVC. Use of salbutamol as rescue therapy was comparable during add-on therapy of formoterol once daily and formoterol twice daily.
Fig. 5. Tiotropium 18 μg once daily (TIO qd), formoterol 12 μg twice daily (FORM bid) and tiotropium 18 μg + formoterol 12 μg once daily (TIO + FORM qd) FEV1 AUCs calculated using the trapezoidal rule divided by the corresponding observation time to report in ml units. ap < 0.05 versus formoterol; bp < 0.05 versus tiotropium. (Data from van Noord et al51.)
The best strategy for adding long-acting β2-agonists and long-acting anticholinergics
These findings raise an important question. What is the best strategy for adding long-acting β2-AR agonists and long-acting anticholinergics in COPD? Some studies have examined various strategies for adding short-acting β2-AR agonists and anticholinergics in COPD. Unfortunately, they have provided conflicting results. Rennard52 has correctly stressed that this may depend on the nature of the circumstances of the patient at the time when the study was carried out and may depend on the design by which the studies were conducted and the drugs administered.
In one of our previous trials, which was the first to our knowledge that compared a long-acting β2-AR agonist and an anticholinergic agent given by sequential inhalation at the recommended dosages, we documented that the sequential administration of formoterol and oxitropium bromide induced an improvement in pulmonary function in a population of COPD patients similar to that examined in the present trial53. However, prior administration of the long-acting β2-AR agonist allowed a response to the anticholinergic drug, which was higher than that observed when inhalation of oxitropium was preceded by that of formoterol.
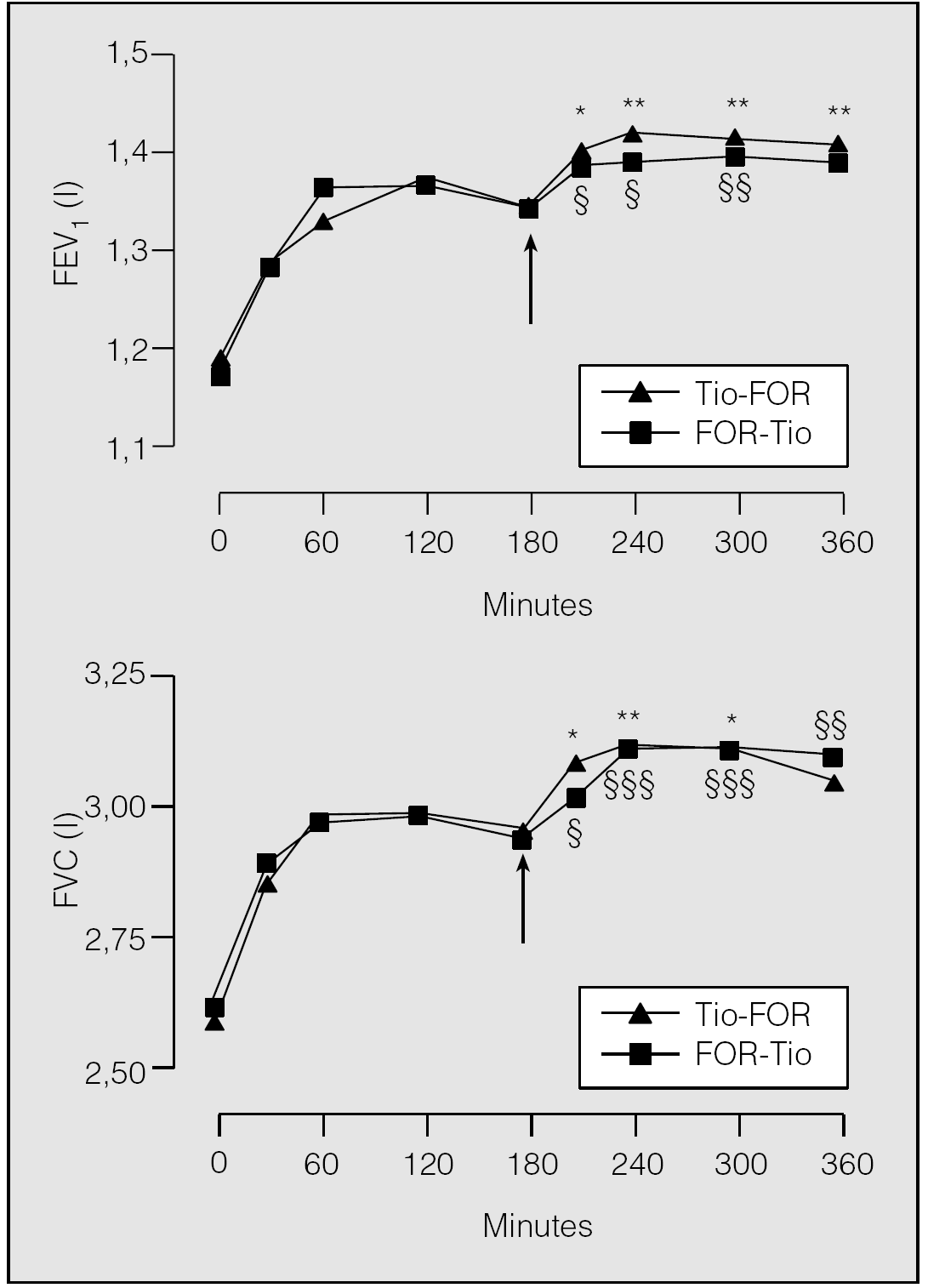
In order to explore whether this finding is true even when tiotropium is used instead of oxitropium, we have examined the potential of an additive effect of a recommended dose of second long-acting bronchodilator (tiotropium or formoterol) in COPD patients under regular treatment with a long-acting bronchodilator of a different class (formoterol or tiotropium, respectively). We conducted a randomized, crossover trial in 20 patients with 18 μg tiotropium once daily and 12 μg formoterol twice daily over a five-day period for each drug, with a ten-day washout period54. At the end of each period, patients inhaled both drugs separated by 180 min in alternate sequence. Thirty minutes after inhalation of the last dose of tiotropium, there was a statistically significant increase of 0.099 l (95% CI, 0.062-0.138) in FEV1 over baseline (p < 0.0001) (fig. 6). The same was observed after inhalation of the last dose of formoterol (0.166 l; 95% CI, 0.064-0.168; p < 0.0002). The mean maximal change in FEV1 over baseline was 0.226 l (0.154-0.298) in group A (regular tiotropium and add-on formoterol) and 0.228 l (0.165-0.291) in group B (regular formoterol and add-on tiotropium). The mean maximal change in FEV1 over pre-inhalation of the second drug value was 0.081 l (95% CI, 0.029-0.133) after tiotropium → formoterol and 0.054 l (95% CI, 0.016-0.092) after formoterol → tiotropium. The mean maximal change in FVC over baseline was 0.519 l (95% CI, 0.361-0.676) in group A and 0.495 l (95% CI, 0.307-0.683) in group B. The mean maximal change in FVC over pre-inhalation of the second drug value was 0.159 l (95% CI, 0.048-0.270) after tiotropium-formoterol and 0.175 l (95% CI, 0.083-0.266) after formoterol → tiotropium.
Fig. 6. Mean response curves following the sequential inhalation of tiotropium (Tio) and formoterol (For) for FEV1 and FVC. Arrow indicates the administration of the second drug. *p < 0.05; **p < 0.01; ***p < 0.001 in the sequence tiotropium → formoterol. §p < 0.05; §§p < 0.01; §§§p < 0.001 in the sequence formoterol → tiotropium. (Data from Cazzola et al54.)
These results suggest that supplementing a second different long-acting bronchodilator to a regularly administered long-acting bronchodilator seems to be to the patient's advantage in terms of bronchodilation. We cannot exclude that the greater bronchodilatory response that we observed when a second bronchodilator was given after the first one may be justified by a carry over effect, considering that both formoterol and tiotropium are long-lasting bronchodilators. Nonetheless, it is well know that the mean peak bronchodilation with both formoterol and tiotropium in COPD patients is reached after two to three hours43 and we have documented that the addition of a second bronchodilator three hours after the inhalation of the first agent, could amplify the maximum bronchodilation of the first agent. This result seems to be important because it indicates the possibility that a patient who is unable to perceive bronchodilation or must perform an exercise could use a second long-acting bronchodilator that will assure a long-lasting effect. In any case, it must be highlighted that significant improvement in pulmonary function has been achieved by adding tiotropium or formoterol at the recommended dosages in patients already in regular treatment with formoterol or tiotropium, respectively, with no statistically significant difference between the different sequences. This finding supports Rennard's opinion3 that treatment can be initiated with an agent from any of the available classes. If symptomatic control is inadequate, an agent from another class can be added.
Conclusions
At present time, there is no clear documentation that tiotropium is superior to formoterol or the contrary. At the recommend doses for COPD therapy, formoterol twice daily and tiotropium once daily induce comparable night time bronchodilation after regular treatment49.
A combination of tiotropium and a long-acting β2-AR agonist is more effective than single drugs alone in inducing bronchodilation and bronchodilator-mediated symptom benefit in patients suffering from COPD42,43,49,51. Add-on therapy of formoterol in the morning to maintenance therapy with tiotropium significantly improves FEV1 and IC for more than twelve hours in patients with moderate-to-severe COPD51. Add-on therapy of a second formoterol dose administered in the evening produces a further increase in average FEV1 and IC, but not in trough IC51. All these findings support, in our opinion, the possibility of combining tiotropium and formoterol, and likely salmeterol, in patients suffering from stable COPD, but exclude the once-daily administration of the two drugs in combination within a single inhaler. It must be determined yet whether tiotropium is more effective when administered in the evening compared with morning dosing, in view of the circadian variation of bronchial tone.
Because long-acting β2-AR agonists are given twice daily but tiotropium bromide is required only once daily, the challenge is now to develop a combined inhaler that can be employed on a once daily basis. The incorporation of once daily dosing is an important strategy to improve compliance since it is a regime preferred by most patients.
Correspondencia: M. Cazzola.
Via del Parco Margherita, 24. 80121 Napoli. Italy.
E-mail: mcazzola@qubisoft.it