Lung transplant recipients (LTR) are at significantly higher risk for cytomegalovirus(CMV) infection than other solid organ transplants (SOT).1 The direct and indirect effect of this infection increases morbidity and mortality in LTR. Universal antiviral prophylaxis for between 6 and 12 months depending on the recipient's serology is recommended.1,2 However, this prolonged use has associated side effects, often requiring prophylaxis discontinuation. Several authors have proposed the Quantiferon®-CMV assay to stratify the risk of CMV disease in SOT, which could help to individualize the duration of antiviral prophylaxis.2–7 However, its role is not well-defined in CMV-seropositive LTR (LTR+)8 who accounts for 80–90% of LTR in Spain. It has been reported that 20% of LTR+ patients with high Quantiferon®-CMV values develop CMV infection.6,8 A plausible explanation for this observation could be that a high immunosuppression inhibits other pathways of the immune response against CMV. Immunosuppression intensity can be measured by Immuknow® assay.9 We hypothesized that the risk of CMV infection in LTR+ could be stratified based on Quantiferon®-CMV and Immuknow® assays.
We studied the risk of significant CMV infection or disease (SICD) between stopping prophylaxis (6 months post-transplant) and study end (12 months post-transplant) according to Quantiferon®-CMV and Immuknow® results at the time of stopping prophylaxis. This was a prospective, observational, multicenter study carried out in 7 centers.
Patients were prospectively enrolled between January 2014 and April 2015. Eligible patients were those ≥18 years old with positive CMV serology pre-transplant who survived for more than 90 days.
Data were collected prospectively and CMV DNA loads were performed during scheduled visits at 3, 6, 7, 8, 9, 10, 11 and 12 months post-transplant. Quantiferon®-CMV and Immuknow® assay were measured at 3, 6, 8, 10 and 12 months post-transplant. Clinicians were blind to assay results.
Quantiferon®-CMV assay (Cellestis, a QIAGEN Company) has been validated to detect CMV-specific immune response by measuring the amount of IFN-γ produced by CD8+ T-cells after ex vivo stimulation with peptides that simulate CMV proteins. Quantiferon®-CMV assay was considered reactive when IFN-γ was ≥0.2IU/mL.3 Indeterminate results (3.4% of assays performed) were not included in the analysis.
The intensity of immunosuppression was assessed by measuring cellular immune function using the Immuknow® assay (Cylex Inc.), which evaluates intracellular ATP levels in CD4+ T-cells. Immuknow® values were low, intermediate or high when ATP was <225, 225–525 or ≥525ng/mL, respectively.10
CMV prophylaxis duration was 6 months. All patients received CMV prophylaxis consisting of intravenous ganciclovir following surgery. Once oral intake was restarted, this was switched to oral valganciclovir 900mg once daily (dose adjusted to renal function) until 6 months post-transplant. Diagnosis of CMV infection/disease was based on established criteria.10 CMV infection was considered significant when CMV DNAemia was higher than 1000copies/mL.
The study was approved by the institutional review board of Hospital Vall d’Hebron (EPA(AG)47/2013). Clinical Trials.gov number NCT02076971.
Seventy-nine (85.9%) patients stopped CMV prophylaxis at 6 months as planned; 13 (14.1%) patients stopped between 3 and 6 months due to adverse reactions.
The proportion of patients free from SCID at 12 months was 78.3% (95% CI, 70.6%–87.4%). No differences were observed between patients who developed SICD and those who did not (Table 1). Twenty-eight cases of SCID were reported in a total of 20 patients (21.7%). No patients developed significant CMV DNAemia while on prophylaxis, and just 2 patients had CMV DNA loads <1000copies/mL, which resolved spontaneously. No patients died of CMV disease. Two patients died due to acute renal failure and septic shock respectively.
Clinical and demographic characteristics of patients who developed significant CMV infection (CMV DNA copies >1000mL–1) or disease and those who did not.
| CMV infection/disease (N=20) | No CMV infection/disease (N=72) | p | |
|---|---|---|---|
| Sex (M/F) | 14 (70%)/6 (30%) | 42 (58.3%)/30 (41.7%) | 0.441 |
| Age (years)(mean±SD) | 57.18±11.32 | 54.28±11.43 | 0.317 |
| Underlying disease | 0.384 | ||
| Pulmonary fibrosis | 11 (55%) | 23 (31.94%) | |
| COPD/emphysema | 2 (10%) | 22 (30.55%) | |
| Cystic fibrosis | 1 (5%) | 9 (12.5%) | |
| Pulmonary hypertension | 5 (25%) | 7 (9.72%) | |
| Other | 1 (5%) | 11 (15.27%) | |
| Type of transplant | 0.64 | ||
| Unilateral | 11 (55%) | 22 (30.5%) | |
| Bilateral | 9 (45%) | 50 (54.34%) | |
| Immunosuppressive induction | 0.211 | ||
| No | 12 (60%) | 31 (43.06%) | |
| Yes | 8 (40%) | 41 (56.94%) | |
| Tacrolimus trough level at 6 months post-Tx (mean±SD) | 10.87±2.77 | 12.82±5.20 | 0.132 |
| Donor CMV serology | 0.338 | ||
| Positive | 17 (85%) | 49 (68.05%) | |
| Negative | 2 (15%) | 14 (19.45%) | |
| Adverse reactions per patient (mean±SD) | 2.29±1.38 | 1.47±0.61 | 0.177 |
| Acute cellular rejection per patient (mean±SD) | 1.33±0.71 | 1.08±0.28 | 0.150 |
| Opportunistic infections (mean±SD) | 1.62±0.87 | 2.10±1.17 | 0.480 |
| Respiratory function at 6 months post-Tx (mean±SD) | |||
| FVC (L) | 2.96±1.18 | 2.67±0.80 | 0.319 |
| FEV1 (L) | 2.29±0.78 | 2.02±0.66 | 0.188 |
| FEV1/FVC (%) | 79.08±11.23 | 76.95±14.94 | 0.503 |
| Lymphocyte total count at 6 months (10*9L–1) (mean±SD) | 2.00±0.10 | 1.97±0.239 | 0.321 |
| Glomerular filtration rate (mL/min) at 6 months (mean±SD) | 67.75±27.12 | 75.58±29.79 | 0.340 |
At the first test (3 months post-transplant), Quantiferon®-CMV assay was reactive in 69 (78.4%) patients and non-reactive in 19 (21.6%), with a median IFN-γ of 1.07IU/mL (IQR: 0.29–10.57IU/mL.) At the last test (12 months post-transplant), 73 (84.9%) patients had a reactive assay, and 13 (15.1%) had a non-reactive assay, with a median IFN-γ of 3.87IU/mL (IQR: 0.58–27.49IU/mL). Median Quantiferon®-CMV values increased over time (p=0.001).
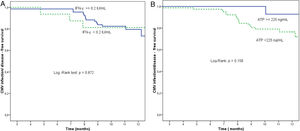
At the end of prophylaxis, Quantiferon®-CMV assay was reactive in 70 of 86 patients (81.4%) and non-reactive in 16 (18.6%). The proportion of patients free from SCID at study end was 78.6% (55/70) in the group with reactive Quantiferon®-CMV at the end of prophylaxis and 81.3% (13/16) in the non-reactive group (p=0.872) (Fig. 1).
(a) Proportion of patients free from significant CMV infection (CMV DNA copies >1000mL–1) or disease according to Quantiferon®-CMV assay, between the end of prophylaxis and the end of the study. Reactive Quantiferon®-CMV (IFN-γ ≥0.2IU/mL, continuous line) vs. non-reactive (IFN-γ <0.2IU/mL, dashed line). (b) Proportion of patients free from significant CMV infection (CMV DNA copies >1000mL–1) or disease according to Immuknow® assay result from the end of prophylaxis to the end of the study. Intermediate/high Immuknow® value (ATP ≥225ng/mL, continuous line) vs. low value (ATP <225ng/mL, dashed line).
At the first test (3 months post-transplant) Immuknow assay was low in 67 (72.8%) patients and intermediate in 25 (27.2%); no patients had high values. The median ATP value was 137.86ng/mL (IQR: 75.50–237.02ng/mL). At the last test (12 months post-transplant) 56 (66.7%) patients had low values, 27 (32.1%) had intermediate values and 1 patient had high values (1.2%), with a median ATP of 177.50ng/mL (IQR: 125.24–254.65ng/mL). Median ATP increased over time (p=0.003).
At the end of prophylaxis, 14 of 91 (15.4%) patients had an intermediate Immuknow® value, 77 (84.6%) had a low value, and none had a high value. The proportion of patients free from SCID at study end was 92.9% (13/14 patients) for those with intermediate values at the end of prophylaxis and 76.6% (59/77 patients) for those with low value (p=0.158) (Fig. 1).
At the end of prophylaxis, no patients had non-reactive Quantiferon®-CMV with intermediate/high ImmuKnow® values. Sixteen (18.8%) patients had non-reactive Quantiferon®-CMV and a low ImmuKnow®. Fifty-six (65.9%) had a reactive Quantiferon®-CMV and a low ImmuKnow®, and 13 (15.3%) had a reactive Quantiferon®-CMV and an intermediate/high ImmuKnow® value. None of these 13 patients developed SICD, compared to 75% of the remaining patients (p=0.056)
The predictive value of both assays was not statistically significant. Quantiferon®-CMV showed an AUC of 0.571 (95% CI, 0.411–0.732; p=0.344) with a sensitivity of 16.7% (95% CI, 4.4%–42.3%) and a specificity of 80.9% (95% CI, 69.2%–89.0%). The positive predictive value (PPV) was 18.8% (95% CI, 5.0%–46.3%) and the negative predictive value (NPV) was 78.6% (95% CI, 66.8%–87.1%). For ImmuKnow®, an AUC of 0.45 (95% CI, 0.29–0.61; p=0.514), a sensitivity of 94.7% (95% CI, 71.9%–99.7%), a specificity of 18.1% (95% CI, 10.3%–29.3%), a PPV of 23.4% (95% CI, 14.8%–34.7%) and a NPV of 92.9% (95% CI, 64.2%–99.6%) were observed.
Quantiferon®-CMV assay has been proposed as a potential tool for predicting CMV infection in SOT after stopping CMV prophylaxis4,6,7,12 and for helping personalize CMV prophylaxis.5 However, most published studies include both seropositive and seronegative patients or/and a small number of lung transplants which makes it difficult to draw conclusions for LTR+. To our knowledge, our study is the first to focus on LTR+ alone. Unlike other SOT, we found no difference in the rate of SCID between patients with reactive and non-reactive Quantiferon®-CMV assays in the months after stopping prophylaxis (around 20% in both groups). Therefore, Quantiferon®-CMV was not useful for risk stratification in this population. Alternate biomarkers such as IFN-γ-CMV ELISPO remain under investigation.13
Little is known about the value of the Immuknow® assay for CMV risk stratification in LTR. Published studies have focused on overall infections in SOT but not specifically CMV infection.11,14–18 Although some studies have suggested its utility for identifying patients at risk of CMV infection in SOT.19,20 In the present study, only 7% of patients with high/intermediate values developed SCID versus 25% of patients with low levels. Therefore Immuknow® could help to individualize the prophylaxis length and CMV DNAemia surveillance. However, differences did not reach statistical significance (p=0.158) and additional studies are needed to confirm this purpose.
Finally, most LT+ had specific immune response to CMV (80% of patients with reactive Quantiferon®-CMV), but the majority were also heavily immunocompromised (70% with low Immuknow® values). This may be the reason why 20% of patients with specific immune response to CMV develop SCID. In fact, no patient with specific immune response and who was not heavily immunosuppressed developed SICD. On the other hand, the observed increase in Quantiferon®-CMV and Immuknow® values over time, probably indicates a certain recovery of immunocompetence.
In conclusion, Quantiferon®-CMV assay was not useful for risk stratification of SCID in LTR+. However, Immuknow® assay may be useful for this purpose, but additional studies are needed to confirm this.
FundingThis work was supported by Roche Farma SA. Madrid, Spain.
Conflict of interestThe authors declare no conflicts of interest.
The authors thank Sonia López and Rosa Llòria and for their assistance and technical help and Núria Pajuelo for her work on the statistical analysis.