In recent years, we have witnessed a paradigm shift based on the development of new technologies, which has extended to the field of healthcare, research, and clinical management. We are currently undergoing an era of disruptive innovation, which will bring about a qualitative and radical change in many facets of our activity as physicians. In medicine, disruptive innovation is already upon us. New technologies for the diagnosis and treatment of certain diseases have been developed. Furthermore, artificial intelligence (AI) has become an aid to healthcare practice and clinical research. Though clinical knowledge will never be fully replaced by technology alone, it is peremptory to use the real-world information that these new technologies have to offer, as an aid to clinical practice. In this scenario, the present editorial focuses on the diagnostic and prognostic utilities of AI regarding pneumological disorders, both on the patient level and from a global healthcare perspective.
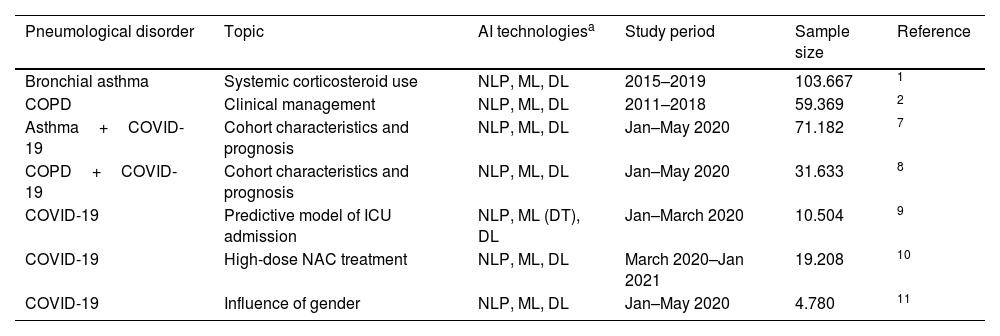
A relevant example of the utility of AI is reflected in a recent study focusing on patients with bronchial asthma that were being treated with systemic corticosteroids.1 By analyzing electronic health records (EHRs) through natural language processing (NLP), a large pool of recent information (from 2015 to 2019) from more than 100,000 patients was used to provide a descriptive snapshot of this population. Patients with bronchial asthma treated with systemic corticosteroids were older, with a higher prevalence of hypertension, dyslipidemia, diabetes, obesity, depression, and hiatus hernia. Furthermore, prescription rates of systemic corticosteroids were also described, which reached 39.6% of the general population with bronchial asthma in 2019, mostly prescribed in primary care. Systemic corticosteroid use is associated with a greater number of adverse events including Cushing syndrome (OR=7.8) and osteoporosis (OR=4.6); therefore, this study raised a red flag to reconsider their frequent use.
Importantly, the patients included in real-world studies reflect their greater complexity compared to participants in clinical trials, who are selected according to stringent inclusion and exclusion criteria, limiting their applicability in real-life settings. In the context of chronic obstructive pulmonary disease (COPD), a recent Big Data analysis of more than 50,000 patients over 40 years old reflected that clinical management was undertaken mostly in primary care and pneumology departments, but also in internal medicine and geriatrics.2 In-hospital mortality and hospital admissions and durations of stays were also described. This portrait of the current state of COPD management in Spain shows that, though multiple national and international clinical guidelines, care plans, and national strategies have been developed since 2011, their impact on critical aspects of COPD management has been practically marginal.2 Only through these novel technologies, which allow us to monitor these strategies in real time, will we be able to implement improvement plans realistically.3 Otherwise, disease management will have to continue relying on registries or audits that require long periods of time, involve many clinicians and resources, and often yield information that is partial, biased, or outdated upon its completion.4–6
In addition to exploiting large datasets, another advantage of AI techniques is that they are deployed faster than classical clinical studies. In this sense, the recent COVID-19 pandemic presented a challenge regarding urgency to obtain data during early stages, to aid in diagnosis, treatment, and prognosis. Making use of the digitalized datasets, in a very short time it was possible to provide answers to overarching questions of COVID19 in patients with chronic pneumological diseases, such as asthma or COPD.7,8 Data collected during the first COVID-19 wave (January to May of 2020) revealed that the proportion of patients with COVID-19 in the COPD population (2.51%) and in the population of patients with asthma (1.41%) was significantly higher than in comparable control groups (0.86% and 1.16%, respectively).7,8 Patients with either asthma or COPD and concomitant COVID-19 were also significantly older, smoked more frequently, and presented more comorbidities. Interestingly, though patients with COPD and COVID-19 showed higher rates of hospitalization and mortality, patients with asthma and COVID-19 treated with biologics and inhaled corticosteroids showed relatively low COVID-19-related hospitalizations. Though some of these results are now common knowledge, AI techniques were at the frontline of high-speed epidemiological research when information was scarce. These and other early data helped to determine which patients were at higher risk of developing COVID-19.
AI techniques such as machine learning can also be used to develop predictive models of disease prognosis. Still within the context of the first wave of COVID-19, a predictive model was developed to explore the factors that predict intensive care unit (ICU) admission of patients with COVID-19.9 Overall, the combination of age, fever, and tachypnea was predictive of ICU admission. Furthermore, the study found that COVID-19 patients under 56 years of age without tachypnea and fever below 39°C (or >39°C without respiratory crackles) were not admitted to the ICU, since they did not develop severe COVID-19. Conversely, those aged 40–79 years who had tachypnea and had delayed their visit to the emergency department were at higher risk of ICU admission.
More specific questions regarding COVID-19 and the impact of treatments or the influence of gender were also tackled. One study focused on treatment found that together to corticosteroids and enoxaparin, N-acetylcysteine (NAC) administered orally in high doses (600mg every 8h) might have a favourable impact on survival when added to the standard of care.10 These studies generate hypotheses, which in the case of drugs, obviously must be validated in controlled clinical trials. In fact, NAC use did not reduce hospitalization stays, ICU admissions, nor use of invasive mechanical ventilation. As for gender, 51% of COVID-19 patients studied were female, but showed significantly more frequent symptoms upon diagnosis such as headache, anosmia, and ageusia.11 Blood tests and chest X-rays were performed less on females than males, and females showed lower hospitalization and ICU admission rates. These large-scale datasets analyzed with NLP and machine learning revealed both prognostic and hospital management insights that otherwise would have required lengthy and prospective clinical trials (Table 1).
Studies using AI technologies in pneumological disorders.
| Pneumological disorder | Topic | AI technologiesa | Study period | Sample size | Reference |
|---|---|---|---|---|---|
| Bronchial asthma | Systemic corticosteroid use | NLP, ML, DL | 2015–2019 | 103.667 | 1 |
| COPD | Clinical management | NLP, ML, DL | 2011–2018 | 59.369 | 2 |
| Asthma+COVID-19 | Cohort characteristics and prognosis | NLP, ML, DL | Jan–May 2020 | 71.182 | 7 |
| COPD+COVID-19 | Cohort characteristics and prognosis | NLP, ML, DL | Jan–May 2020 | 31.633 | 8 |
| COVID-19 | Predictive model of ICU admission | NLP, ML (DT), DL | Jan–March 2020 | 10.504 | 9 |
| COVID-19 | High-dose NAC treatment | NLP, ML, DL | March 2020–Jan 2021 | 19.208 | 10 |
| COVID-19 | Influence of gender | NLP, ML, DL | Jan–May 2020 | 4.780 | 11 |
NLP and ML in these studies was deployed using EHRead® technology, which extracts information from the free text of EHRs using NLP, ML, and DL, converting text to clinical terms based on SNOMED-CT terminology. The study periods run from January 1st of the first year to December 31st of the last year, unless otherwise indicated. Sample size quantifies patients meeting all inclusion and no exclusion criteria, prior to subgrouping.
AI: artificial intelligence; COPD: Chronic Obstructive Pulmonary Disease; COVID-19: Coronavirus Disease 2019; DL: deep learning; DT: decision trees; EHR: electronic health record; ML: machine learning; NLP: natural language processing; SNOMED-CT: Systematized Nomenclature of Medicine-Clinical Terms.
AI techniques such as NLP, machine learning, or deep learning allow approaches that can give us answers that we cannot currently obtain with traditional research. These techniques, applied to increasingly digitized clinical information, are going to be fundamental for diagnostic, prognostic, and health management of disease, including pneumological disorders. Furthermore, there is an ongoing need to work with reliable, high-quality information that extends beyond population-based averages and becomes relevant for specific groups or even individual patients. In this shift towards personalized medicine, Big Data and AI play a leading role. Approaches that involve personalized medicine avoid unnecessary treatments, helping to improve not only individual patients’ health, but also providing crucial information towards decision-making in the context of healthcare systems.