Bronchiectasis is a highly heterogeneous chronic airway disease characterized by multiple etiologies, diverse clinical manifestations, different microbiological profiles, a variety of radiological and functional patterns, and overlapping conditions. All these pose major therapeutic challenges to the clinicians.1 Clinicians and patients are understandably frustrated as there are few evidence-based treatments for bronchiectasis. An example of the paucity of evidence is that in 2008, the Journal published the first widely recognized guideline for bronchiectasis in Spain, when the negative findings of recombinant DNAse represented the only multicenter international trial of a therapy in non-CF bronchiectasis to develop evidence-based guideline.2
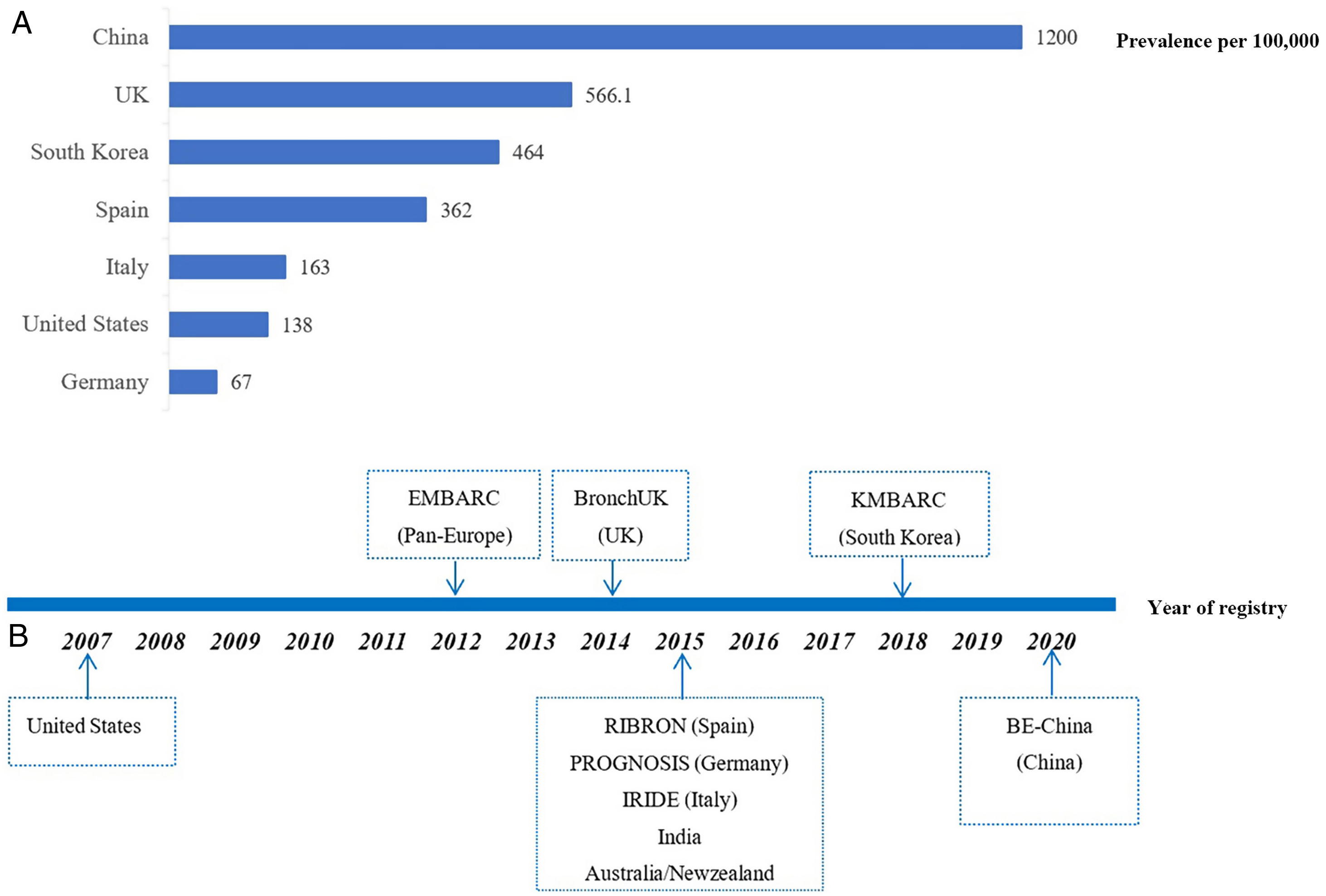
Historically, bronchiectasis was regarded as an orphan disease, defined by the European regulators as a disease with a prevalence of less than 50 per 100,000 individuals. While the improving access to chest computed tomography (CT) scans and the aging population have raised the awareness of bronchiectasis, the global prevalence of bronchiectasis has been increasing over time. In fact, the prevalence estimates of bronchiectasis significantly differ worldwide, ranging from 67 per 100,000 in Germany to 566 per 100,000 persons in UK (Fig. 1A),3–6 possibly reflecting the differences in study design, disease definition and data collection as well as geographic differences in disease burden. Nevertheless, these figures far exceed the threshold of an orphan disease. The epidemiological data of bronchiectasis in China remains largely unclear, but an urban population-based cross-sectional survey between 2002 and 2004 showed that the prevalence of patient-reported physician-diagnosed bronchiectasis among individuals aged ≥40 years was 1200 per 100,000 persons,6 with an estimated at least 16 million patients with bronchiectasis in China. Therefore, bronchiectasis is associated with substantial healthcare costs not only at a national but also at international level. Unfortunately, to date, there are still no approved drugs for use in bronchiectasis to halt the progression of the disease.
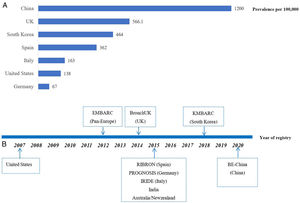
During the last decade, bronchiectasis has experienced a renaissance with an increase in clinical research due to the renewed interests globally. Large-scale registries have been set up in Pan-European Countries (EMBARC) and in the United States as well as in individual countries including Spain, Italy, Germany, India and South Korea (Fig. 1B) mainly since 2012, allowing for the close collaborations between expert clinicians and researchers of the involved countries and centers.7–9 Using data of these registries, physicians now have more data about the demographics, the aetiological and microbiological profiles, and the burden of disease such as symptoms, comorbidities, quality of life (QoL) and frequency of exacerbations.10,11 Epidemiological data have ascertained the key role of Pseudomonas aeruginosa, concomitant with COPD or asthma on the poor outcome.10,11 Recognition of the negative impact of frequent exacerbation on clinical outcomes highlights the importance of preventing exacerbation in clinical practice, of which are emphasized by international bronchiectasis guidelines.10 Furthermore, confirmation of eosinophilic inflammation as one of key features in a subgroup of patient with bronchiectasis may help identify individuals who would benefit from eosinophil-targeted therapy.12 All these key advances in the past 10 years were made through multicenter, international collaboration based on established registry platforms, mainly from EMBARC, which could not be achieved by single-center, small sample size studies.
However, almost all these influential studies have enrolled patients from western countries. Data regarding the disease burden and demographics of patients outside of western countries are scarce and may differ substantially. Emerging data have shown significant geographic and ethic variation of the disease globally, such as post-tuberculosis as a common cause of bronchiectasis in Asia, a high prevalence of NTM in United States, and a high prevalence of bronchiectasis in indigenous populations.3 This suggests an urgent need for research to be done in different patient populations and to carefully phenotype the patients.
The disease burden of bronchiectasis in China is huge, especially in light of its population. Unfortunately, to date, reports of bronchiectasis in China are mainly retrospective, single-center, cross-sectional studies with relatively small sample size, and there are few large multicenter clinical trials and prospective multicenter cohort studies with linkage to the clinically important outcome measures primarily including Chinese bronchiectasis patients.13–15 Thus, there is an imperative need to establish the Chinese bronchiectasis registry and research collaboration (BE-China) to compensate these gaps and contribute to the overall knowledge evolving worldwide. According to the study protocol, the BE-China is an ongoing prospective, longitudinal, multi-center, observational cohort study aiming to recruit a minimum of 10,000 patients, which was formed in January 2020 in China. We are collecting the comprehensive data of patients, including medical history, aetiological testing, lung function, microbiological profiles, radiological scores, comorbidities, mental status, and QoL at baseline. Patients will be followed up for up to 10 years to record the longitudinal data on outcomes (such as lung function, exacerbation, and mortality), treatment patterns and QoL. Meanwhile, biospecimens, if possible, will be collected and stored for further research. Four prospective studies on bronchiectasis are being performed through this platform, generating more and more research proposals. The main objective of BE-China is to describe the cross-sectional spectrum and natural history of bronchiectasis by long-term follow-up, as well as to reveal the phenotypes/endotypes of bronchiectasis by integrating the microbiome, proteome, genome and transcriptome with detailed clinical data, and facilitate large randomized controlled trials and evidence-based clinical practice in China.
Several challenges merit discussion. Since the patients will be mainly enrolled from secondary and tertiary hospitals, it appears that patients may not represent all spectrums of bronchiectasis in China. However, the referral system in China is not strict, and patients can go directly to public hospitals for all out-patients’ care, and then be enrolled in the BE-China. Therefore the findings will be generalizable to the patients from other regions of China. In addition, withdrawal of patients and missing data may result in bias and there may be other unidentified or unmeasured confounding factors.
As the saying goes “Better late than never”. We are happy to formally join the global community of health care professionals, scientist, and patients who are trying to end this neglected disease and improve the outcomes for patients through establishing the national bronchiectasis registry in Chinese patients. We believe that the BE-China will not only promote research and high-quality care of bronchiectasis in China, but also facilitate multidisciplinary collaborative research nationally or internationally. It is a promising event for bronchiectasis patients in China – the beginnings of a new template!
FundingNational Natural Science Fund for Distinguished Young Scholar (No. 81925001) and Key Scientific Innovation Project of Shanghai Municipal Education Commission (No. 202101070007-E00097).