A 2022 update of the consensus for severe asthma in adults was recently published in the Open Respiratory Journal.1 This document was drawn up by 15 authors with the participation of 78 expert asthma panellists who validated the recommendations and conclusions of the consensus in a 2-round Delphi process.
This document updates the recommendations published in 2020.2 New developments include a definition of severe uncontrolled asthma (SUCA), as follows: “when poor disease control persists despite treatment over the preceding year with a combination of high-dose inhaled glucocorticoids/long-acting β2 agonists and long-acting anticholinergics, or if maintenance systemic glucocorticoids are required (treatment lasting 6 months per year regardless of dose, or cumulative dose >1g of prednisone or equivalent regardless of duration)”.
Another new feature is the assessment of response to monoclonal antibody therapy (mAB) using the EXACTO multidimensional scale (EXacerbations, ACT, systemic CorticosTeroids and Obstruction-FEV1), with two scores ranging from 0 to 10 or 0 to 7 depending on whether the patient is receiving continuous systemic glucocorticoids (CGC) or not, respectively.3 Furthermore, the SNOT 22 questionnaire and a visual analogue scale are proposed for patients with chronic rhinosinusitis with nasal polyposis. Taken together, these indices provide a useful assessment of single-airway super-responders. Finally, patient satisfaction is assessed using a 0 to 10 scale that measures factors such as efficacy, adverse effects, ease of use, impact on daily activities, and general opinion.
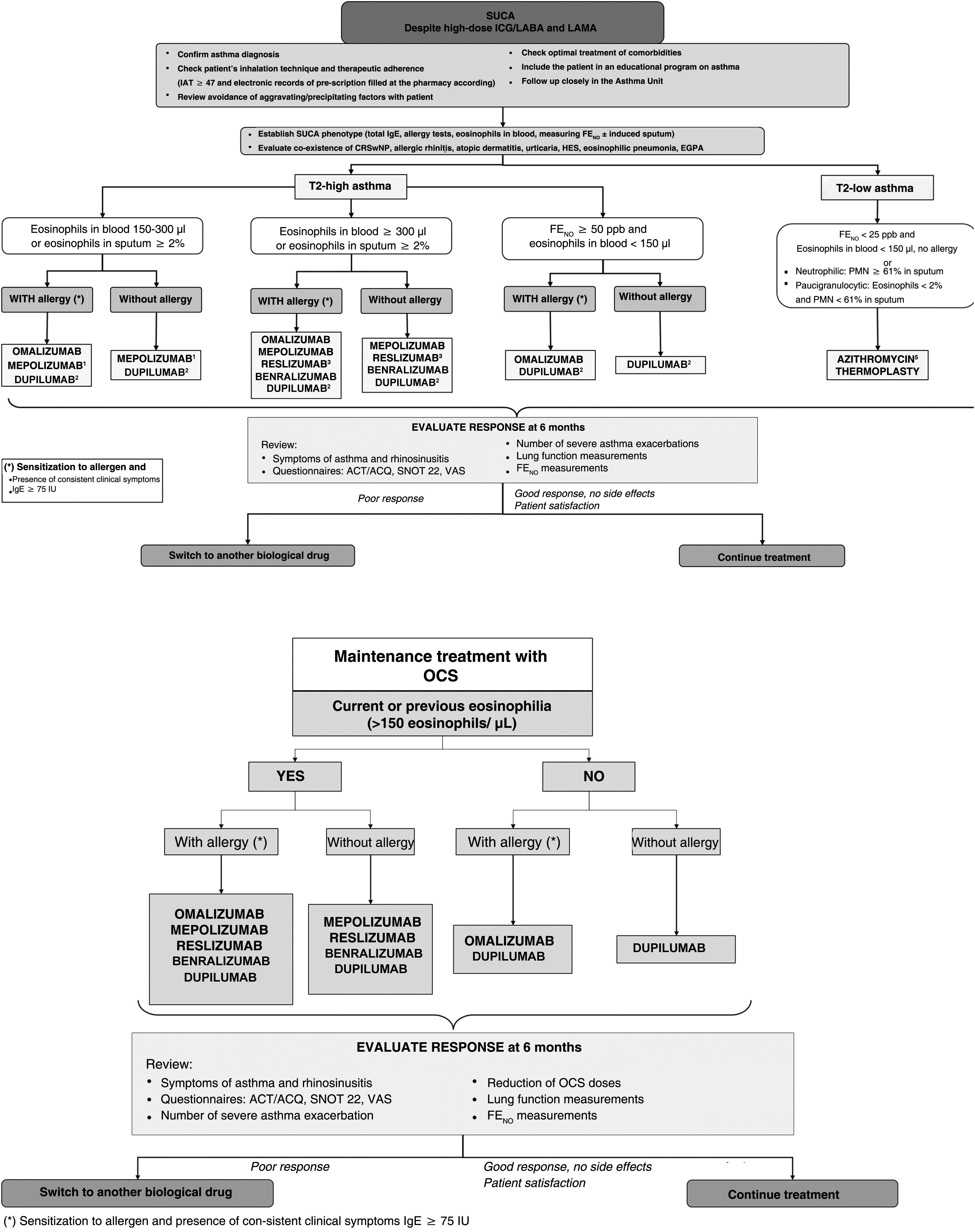
In terms of treatment, after confirming the diagnosis of SUCA and assessing comorbidities, one of 2 new phenotypes will be assigned: T2-high and T2-low asthma. In T2-high asthma, the dichotomy of blood eosinophilia >300/μl and 150–300/μl has been maintained, but a group with FENO >50ppb and eosinophilia <150/μl has been added. The T2-low group includes not only eosinophilia <150/μl, but also FENO values <25ppb, while induced sputum should be obtained for phenotyping if possible (Fig. 1). A specific algorithm for patients receiving CGCs has also been developed, with current or historical eosinophilia levels ≥150/μl being considered significant (Fig. 1). The publication includes guidelines for the withdrawal of oral corticosteroids and new recommendations on smoking, inhalation devices, environmental pollution, and climate change. The effects of SARS-Cov-2 and recommended vaccination guidelines are also addressed.
SUCA treatment algorithm. ACT: asthma control test; ACQ: asthma control questionnaire; CRSwNP: chronic rhinosinusitis with nasal polyposis; EGPA: eosinophilic granulomatosis with polyangiitis; FENO: exhaled nitric oxide fraction; HES: hypereosinophilic syndrome; IAT: inhaler adhesion test; IGC: inhaled glucocorticoids; LABA: long-acting β2 bronchodilators; LAMA: long-acting anticholinergic agents; OCS: oral corticosteroids; PMNs: polymorphonuclear neutrophils; SNOT 22: Sino-Nasal Outcome Test 22; SUCA: severe uncontrolled asthma; VAS: visual analogue scale.
1Mepolizumab is indicated if current Eos ≥150/μl and ≥300/μl in the previous 12 months; this drug is also indicated for eosinophilic granulomatosis with polyangiitis and hypereosinophilic syndrome.
2Dupilumab is indicated if Eos ≥300/μl and/or FENO ≥50ppb and Eos between 150 and 300 and FENO ≥25ppb. At least 3 FENO measurements should be used.
3Reslizumab is indicated if EOS ≥400/μl.
4Compassionate use of omalizumab may be considered in case of IgE levels ≥75U/L and Eos levels <150cells/μL.
5In T2-high asthma, azithromycin may be selected in case of a lack of response, intolerance, or allergic reactions with monoclonal antibodies.
Attention is given to the role of respiratory nursing in patient education, the evaluation of control and therapeutic compliance, mAB follow-up, and the identification and training of patients who may be candidates for self-administration at home.
Finally, the document assesses the organization of asthma care units, the allocation of technical and human resources, accredited training, teaching and research, the importance of a multidisciplinary approach, referral criteria and health outcome indicators.4,5
We hope this revised consensus will be useful to all professionals who treat asthma patients and that it attracts the same interest as the previous reviews.