Introduction
The concept of variation in medical practice refers to the nonrandom variation in the use of hospital resources--such as diagnostic tests, medical treatment or surgical interventions, hospital admissions, length of hospital stay, and others--by populations in geographical areas or among patients in similar clinical situations.1 Variation in medical practice raises pivotal questions regarding quality of care in the broadest sense, including accessibility, appropriateness, effectiveness, costs, and equity. It is known that these variations lead to problems of effectiveness in health care delivery and societal efficiency.2 Practice variations within geographical areas--the most relevant variations, being those that involve large-scale management--have been extensively documented by the Spanish National Health System2-8; however, there are few studies which analyze practice variations among health care professionals, an essential factor in clinical management.
In Spain chronic obstructive pulmonary disease (COPD) is the primary reason for hospitalization in internal medicine and subspecialties and is usually the second most common reason for admission to general hospitals, surpassed only by normal childbirth.9 Although the mean length of hospital stay for COPD patients has decreased in recent years, the rate of COPD admissions is on the rise, probably due to factors such as the progressive aging of the population, hospital characteristics, and variations in medical practice.10,11 Given the heavy impact of COPD on health care systems, variations in the use of resources for treating this disease are highly significant for health care management.12 Moreover, for a specific hospital where supply and demand factors are homogeneous, variations in treating COPD may indicate that optimal health care--as reflected by use of hospital resources and quality of care--is not being delivered. In fact, in a narrowly defined population subject to similar conditions, variations in the use of hospital resources can be attributed only to variations in physician practice or in the severity of patient illness. Once the variables related to severity are controlled for, a finding of persistent variability indicates inefficiencies which should be addressed through health care management.
The aim of this study is to describe the variation in use of resources (number of days of hospitalization and pharmaceuticals use during hospitalization) to treat COPD by physicians at a single hospital, to analyze factors associated with greater or less use of resources and, after controlling for severity of patient illness, to evaluate whether care by different physicians indicates a variation in use of resources.
Material and Methods
Design and Study Population
The population of the present retrospective study was a cohort of patients admitted to the Hospital de Mataró, Catalonia, Spain, from 1996 to 1998 with chronic obstructive bronchitis or emphysema.
We selected all hospital admissions during that period (n=1528) provided the primary diagnosis was chronic obstructive bronchitis or pulmonary emphysema (code numbers 491.20, 492.21, 491.8, 491.9, 492.0, 429.8, and 496 of the International Classification of Diseases, Ninth Revision, Clinical Modification). To assure patient similarity in the study population we excluded patients if their length of hospital stay was less than three days (n=39), if they were younger than 40 years of age (n=27), were admitted to the intensive care unit (n=66), died while hospitalized (n=95), or were treated by physicians with fewer than 20 COPD patients per year (n=356). The last exclusion criterion ruled out substitute physicians or those whose limited experience in treating COPD might contribute to additional variation. A total of 495 cases were excluded (32.4%), leaving 1033 admissions to analyze.
Measurements of Results and Variables Studied
Length of hospital stay was measured and pharmaceutical expenses were calculated in peseta values for 1996 to 1998 and are expressed in euros.* For evaluating the cost of pharmaceuticals, entries of less than €6 (n=2) were excluded.
To control for the differences in severity of patient illness that could justify the variations in consumption of resources, we used the following variables: age; Charlson comorbidity index; other comorbidities especially relevant to COPD (respiratory insufficiency, atrial fibrillation and other arrhythmias, heart failure, and bronchiectasis); arterial partial pressure of oxygen (PaO2), excluding pressures recorded for venous blood, values less than 35 mm Hg (indicative of venous blood), and values greater than 100 mm Hg (indicative of oxygen therapy); oxygen saturation (SaO2), excluding values less than 70 (indicative of venous blood gases) and values greater than 98 (indicative of oxygen therapy); arterial partial pressure of carbon dioxide (PaCO2), excluding pressures recorded for venous blood and values less than 20 mm Hg (indicative of hyperventilation) and excluding records in which the sum of PaO2 and PaCO2 was greater than 130 (indicative of oxygen therapy); pH; serum bicarbonate (HCO3); base excess; and forced expiratory volume in 1 second (FEV1) expressed as a percentage of the predicted value for age and sex.
Sources of Information
We used our hospital admissions Minimum Basic Data Set (MBDS) for identifying the cases and obtaining information on age, sex, diagnosis, length of hospital stay, and discharge status. Since the beginning of the 1990s the Hospital de Mataró has paid particular attention to careful, complete coding of the MBDS and, for the period investigated in the present study, the MBDS includes coded records for 100% of discharged patients, with an average of 4.3 diagnoses per hospital stay, well above the mean of the National Health System, which was about 3 diagnoses per stay during the same period. We supplemented this basic data with information from laboratory databases (gasometry and others) and we manually reviewed medical histories to find the results of FEV1 which were not in the computerized database. Given the characteristics of this study, a retrospective review of computerized data and medical histories, we could not always obtain values for all parameters for all patients.
Confidentiality and Ethical Aspects
Because of the characteristics of the study, ethics committee approval was not required. The database designed for the analysis included no identifying information for the patients except a number linked to the number of the medical history of each case. Measures were taken to prevent access to this information by third parties.
Statistical Analysis
First, we compiled descriptive statistics for the clinical characteristics and gasometry of the patients and for the outcome variables (length of stay and pharmaceutical expense). Given that many variables, including dependent ones, were not normally distributed (generally having asymptotic right-tailed distributions), we used the median as the measure of centrality and the interquartile range (IQR) as the measure of dispersion. (Occasionally, when the medians carried little meaning given that the values were quite similar for all the cases, we used the 5% trimmed mean). For presentation of the descriptive statistics, the cases were stratified in subgroups based on value ranges appropriate to each variable and the corresponding 95% confidence interval calculated by the exact binomial formula. Second, we performed a bivariate analysis to investigate the associations between patient factors (clinical and gasometric) and the outcome variables. We repeated this analysis to evaluate differences among physicians relative to the types of patients treated. We used the Kruskal-Wallis nonparametric test to evaluate the relation between variables. Building on the results obtained from this bivariate analysis and on the conceptual framework previously established to describe the severity of a patient's condition, we developed two multiple linear regression models (one for length of stay and another for use of pharmaceuticals) with the aim of evaluating the differences in these 2 outcome variables related to care by one physician or another, controlling for other variables associated with longer hospitalization or greater use of pharmaceuticals. We used the backward stepwise regression method (construction of a saturated model that includes all variables and then eliminating the nonsignificant ones) with the probability of inclusion at .05 and of exclusion at .10. All calculations were run using the software program STATA® (Stata Corporation, College Station, TX, USA).
Results
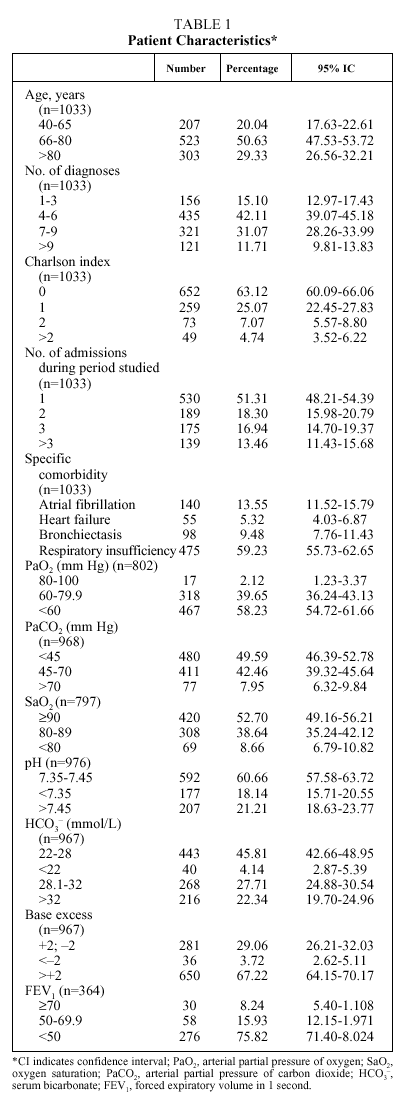
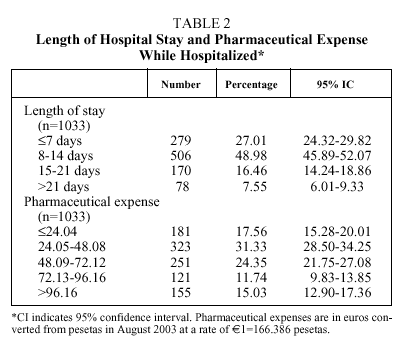
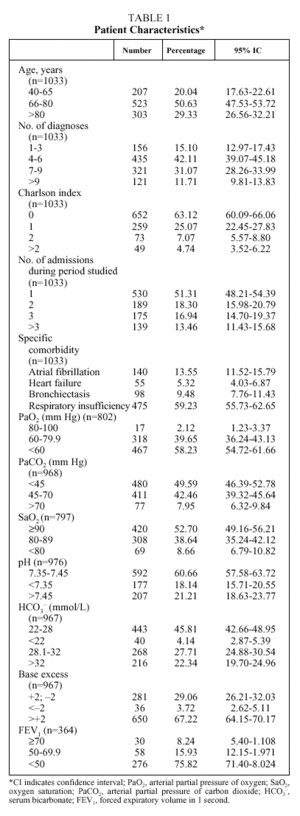
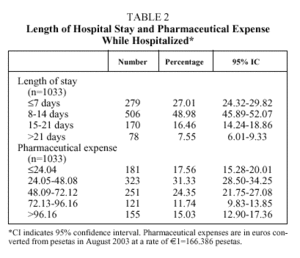
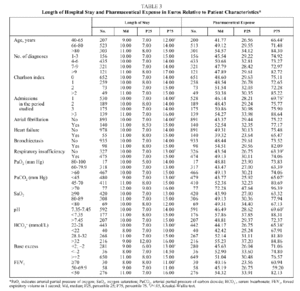
The mean age of the patients was 74 years (median: 76; IQR, 68-82), with more than half between 66 and 80 years (Table 1); numerous associated diseases were diagnosed (median: 6 diagnoses per patient; IQR: 4-8), and 37% of the patients had an associated disease included in the Charlson comorbidity index (mean: 0.57; median: 0; IQR: 0-1). Fifty-eight percent of the patients had respiratory insufficiency (PaO2<60 mm Hg), 50% were hypercapnic (PaCO2>45 mm Hg) and 18.1% presented uncompensated acidosis, although the majority had HCO3 levels and base excess indicating compensation for hypercapnia. The mean length of hospital stay (Table 2) was 11.79 days (median: 10; IQR: 7-14) and the mean expense for pharmaceuticals per admission was €61.87 (median: €48.81; IQR: €29.57-€75.29).
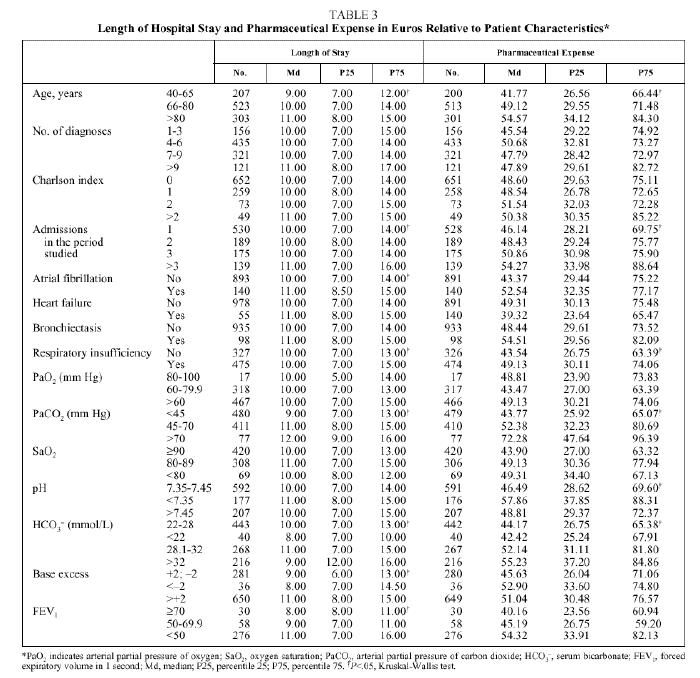
The length of hospital stay (Table 3) increased significantly with the increase in age of the patient, with the number of admissions per patient during the period studied, and with the presence of atrial fibrillation or respiratory insufficiency. The highest pharmaceutical expenses per admission were associated with the same variables--with the exception of atrial fibrillation, for which the increase in expense was not significant. Length of stay was longer for patients whose gasometry indicated ventilatory insufficiency or chronicity and when FEV1 was less than 50%, whereas PaO2, SaO2, and pH showed no relation to length of stay. An increase in pharmaceutical expense was associated with a pH less than 7.35 and with increases in PaCO2 and HCO3.
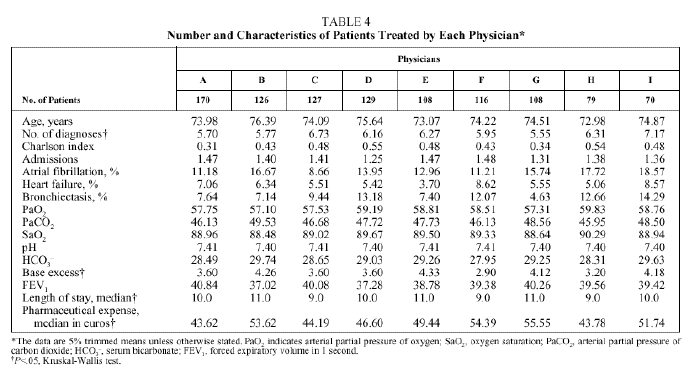
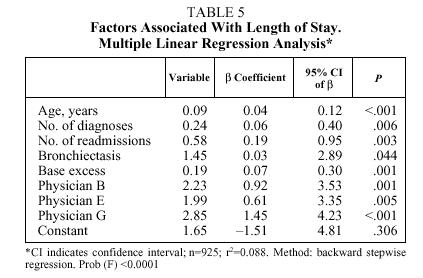
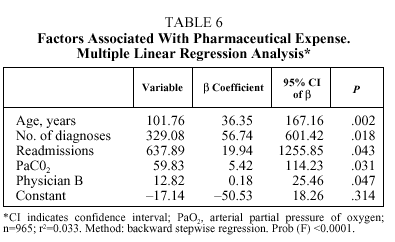
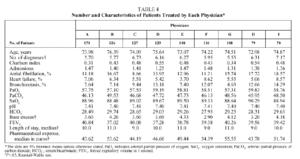
No differences were found in patients treated by each physician (Table 4) with respect to age, pH, PaCO2, PaO2, HCO3, SaO2, and FEV1; however, differences were found with respect to the number of diagnoses per stay (trimmed mean ranging from 5.5 to 7.2 depending on the physician) and base excess (trimmed mean ranging from 2.9 to 4.3 depending on the physician). The median stay ranged from 9 days (physicians C, F, and H) to 11 days (physicians B, E, and G). The median pharmaceutical expense per stay ranged from €43.62 (physician A) to €54.39 (physician F).
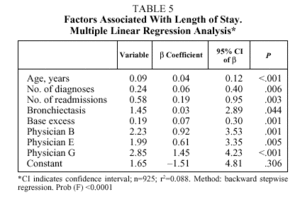
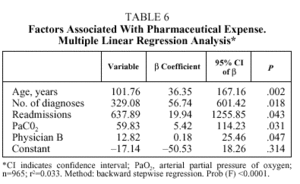
Table 5 details the results of multiple linear regression regarding the length of stay variable (in days). Starting from a constant of 1.65 days, and controlling for the rest of the factors, the length of stay increased 0.09 days with each year of age (starting from 40 years), 0.24 days with each additional diagnosis, 0.58 days with each admission per patient during the period studied (1, 2, 3, and more than 3), and 0.19 days with each unit increase in base excess. After controlling for the effect of these factors, treatment by physician B resulted in 2.2 additional days of hospitalization; physician E, 2 days; and physician G, 2.8 days. As to pharmaceutical expenses (Table 4), starting with a negative constant of €17.13 and controlling for the rest of the factors, each year of age resulted in an increase of €0.61; each additional diagnosis, €1.98; each readmission, €3.83; and each point of PaCO2, €0.36. Only physician B generated a significantly higher expense than the rest (€12.82 more for patients treated by this physician).
Discussion
The results of the present study indicate that, for a normal cohort of uncompensated COPD patients admitted to a single hospital, the length of stay and pharmaceutical expense per stay vary depending on the physician in charge of the case. These variations persist after controlling for other variables and, for some physicians relative to others, result in increases in length of hospital stay of longer than 2 days and increases in pharmaceutical expense of greater than €12.00 (relative increases exceeding 20% in both cases). This variation in practice has significant repercussions on the efficiency of the hospital, given the frequency of COPD admissions, such that treatment by the physician with the shortest median length of stay compared to the physician with the longest meant more than 2000 days of hospitalization avoided and more than €12 020 in pharmaceutical expenses saved.
Although the mean length of stay (11.8 days) of the patients analyzed in the present study is somewhat less than the 12.7 days reflected in a previous study done in the Hospital del Mar,15 in Barcelona, and similar to the 11.9 days of a recent study in the north of Finland,16 or the 11.6 days of a 1999 study in the Hospital la Fe,17 in Valencia, it is significantly greater than the 6.8 days of a study in the Hospital de Requena,12 Valencia, or the 6 to 9 days of studies in Canada, England, Ireland, New Zealand, and the United States,18-23 or the less than 6 and even less than 4 days of hospitalization found in studies of hospitals that apply organizational measures to reduce the length of hospital stay for COPD patients.24-28 Although the type of patient selected for each study partly explains the respective differences in lengths of stay, the marked differences among centers--much greater than the differences among the physicians of a single hospital found in the present study--lead us to conclude that organizational factors specific to each hospital have a greater impact on length of stay than the variation in medical practice within a single hospital. We feel that such factors highlight the need for dissemination of the comparative results of numerous hospitals and for global management of use of hospital resources in order to direct practice style toward more efficient protocols for all physicians--not only the ones who "deviate" from the norm.29
Length of hospital stay was also associated with age, the number of previous admissions, atrial fibrillation, respiratory insufficiency, PaCO2 greater than 70 mm Hg, HCO3 greater than 28 mmol/L, base excess greater than +2, and FEV1 less than 50%. These findings are consistent with those of other studies18,21,30 that associate age, chronicity, and functional status to length of stay; however, we did not find the relation to comorbidity that other studies found.31 We found no studies analyzing variables associated with pharmaceutical expenses that were for the most part comparable to those associated with length of stay.
There are limitations to the present study, as with all studies based on information from retrospective administrative databases. First, a retrospective design implies information gaps and bias; moreover, studies on quality based on such databases show serious drawbacks in almost all areas.32-34 Such drawbacks are probably less serious in the present study due to both the effort of the hospital to improve record coding (reflected in the high number of diagnoses per case relative to other studies) and to the fact that discharge records of all physicians were coded by the same data processing department. However other information of interest such as the presence of respiratory infection at the time of admission, corticosteroid treatment, number of years' duration of illness, home oxygen therapy, etc, were not available and may partly explain the variation among physicians. Although assigning patients to physicians, done in the emergency unit, followed no systematic criterion and although we excluded patients with marked differences in risk factors, both the most critical cases (patients who required immediate admission to the intensive care unit or who died) and the mild to very mild cases (patients who were hospitalized fewer than 3 days), the design does not assure patient similarity for each physician.
As to the variables used to evaluate patient severity of illness, the number of secondary diagnoses is an imprecise variable due to the equal weight given to diverse diagnoses. The difficulty in obtaining a specific scale, beyond recursively deriving one from the same patients as the study population, and the lack of information on significant comorbidities justified the use of the secondary diagnoses but, again, the design cannot assure that the adjustment from such a variable controlled for the real differences in use of resources among patients. In the case of pharmaceutical use, the literature offers little information on predictive factors and we opted to use the same variables as we used for length of stay although it is not obvious that both measures of use of resources should necessarily be related to the same factors.
Quite a few of the analyses performed were run without using corrections for multiple testing, which might have facilitated the identification of some spurious associations, although the associations identified are supported by clinical logic. Groups for some of the bivariate analyses were small, which may have hampered the identification of associations that really existed. Furthermore, the dependent variables and most of the independent variables followed an abnormal distribution, showing a long, right-hand tail approaching logarithmic distribution--as in the case of the mean length of hospital stay. This problem was overcome using nonparametric testing in the bivariate analyses, but that recourse constitutes a limitation in linear regression modeling. Despite this limitation we opted to use measures of centrality for the dependent variables themselves (instead of normalizing them by taking their logarithms) given that following the long tradition of using the mean length of stay in hospital care facilitates understanding the results. Although the β coefficients obtained from these models should be viewed cautiously, it should be pointed out that no change in the variables included in the respective models occurred when we repeated the regression using the natural logarithm of the mean length of stay and pharmaceutical expense.
An interesting aspect of the present study was the combination of various databases from our hospital. Using the convenient MBDS brought advantages such as the low cost of gathering data and the inclusion of all patients with the diagnoses studied. However, these advantages were countered by the lack of available information and by various biases. Nevertheless, we were able to overcome some of these disadvantages by using additional hospital databases, such as laboratory records, from which we obtained the gasometric variables that are key to evaluating patient severity in COPD cases.
As a whole, the present study indicates--albeit with many shades in interpretation due to the shortcomings of the information sources--that differences among physicians are significant regarding length of hospital stay and use of pharmaceuticals, and that these differences persist after controlling for the relative differences in severity of patient illness. In order to confirm such a variation in medical practice more conclusively, prospective studies will be required, although they are unusual in research on the outcome variables we studied except when they are relevant as secondary measurements in clinical trials. In the absence of prospective studies, available information from research of less robust design indicates the need for health care management to develop protocols for modifying the practice style of physicians and of hospital services as a whole (practice guidelines, clinical protocols, organizational changes, etc), especially for a disease with the societal impact of COPD.12,35
Acknowledgments
Special thanks is due to Dr Santiago Bardagí for his help in evaluating the severity of patient illness based on gasometric parameters, to Ms María José Ródenas for her help in the extraction and elaboration of the database; and to Ms Sandra Gea, Ms Cristina Navas, and Ms Etna Moreno for their manual revision of the medical histories.
*Translator's note: Amounts in pesetas have been converted to euros in August 2003 at a rate of 166.386 Spanish pesetas to € 1 and rounded to 2 decimal places.
Correspondence: Dr. S. Peiró.
Fundación Instituto de Investigación en Servicios de Salud.
San Vicente, 112, 3.º. 46007 Valencia. España.
E-mail: speiro@comv.es
Manuscript received January 21, 2003.
Accepted for publication March 18, 2003.