Introduction
Sleep apnea-hypopnea syndrome (SAHS) is a clinical condition characterized by abnormal, repeated, breathing pauses during sleep, secondary to a functional obstruction of the upper airway. Several studies have suggested there is a relation between untreated SAHS and impaired quality of life,1 cardiovascular2-4 and cerebralvascular5 complications, and traffic accidents.6 Untreated SAHS has been shown to raise health care costs7 and could be related to an increase in morbimortality.8
The American Academy of Sleep Medicine9 recently defined SAHS as existing when the apnea-hypopnea index is greater than 5 and associated with relevant clinical symptoms and signs. The Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) defined SAHS as the appearance of abnormal repeated breathing pauses during sleep, caused by a morphophysiologic alteration in the upper airway which leads to its collapse.10 Conventional nocturnal polysomnography (PSG) is the most highly recommended diagnostic method.11-13 However, given the prevalence of SAHS amongst the general public14,15 and the lack of availability of PSG in public health centres in Spain,16 more simplified and less costly systems have been proposed for the initial diagnosis of patients clinically suspected of SAHS. The better known devices are respiratory polygraphic screening devices (RPSD) that measure respiratory rather than neurophysiological variables.17 Their diagnostic yield is less than that of PSG and must be expressed in terms of probability. This means each device on the market must be validated against the PSG as the gold standard, and within the population it will be used in. Moreover, the scientific evidence available, although sparse, indicates more studies are needed on simplified systems which can have a high degree of sensitivity and specificity and complement the use of PSG as the diagnostic method in populations with high prevalence such as patients treated at sleep clinics for suspected SAHS.11
The objective of the present study was to validate the BREAS SC20 (Breas Medical AB, Mölnlyke, Sweden) RPSD for the initial diagnosis of patients suspected of SAHS, using the PSG as a standard of comparison.
Patients and Methods
Participants
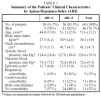
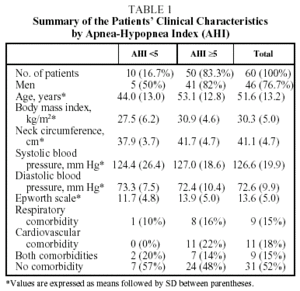
Between November 2001 and August 2002, 70 adult patients were consecutively enrolled at the Sleep Unit of the Hospital Txagorritxu de Vitoria-Gasteiz (Álava, Spain), having been referred from the outpatient clinic of the same unit to the laboratory for a PSG diagnostic test. The patients were clinically suspected of SAHS following an interview with one of the research group experienced in the diagnosis and treatment of sleep disorders. Patients were asked about the occurrence of snoring, breathing pauses during sleep, asphyxia sensations, daytime sleepiness, and other SAHS symptoms. The following information on patient characteristics was collected: sex, age (years), body mass index (kg/m²), neck circumference (cm), systolic and diastolic blood pressures (mm Hg), and cardiovascular and/or respiratory comorbidities. Daytime sleepiness was measured on the Epworth scale validated for the Spanish population.18 Patients referred for PSG for other conditions (epilepsy, narcolepsy, etc.) or who tested as having less than 180 minutes sleep on the PSG or less than 300 minutes on the RPSD were excluded from the study. With the 60 patients finally enrolled in the study, real differences of 0.1 or more in the intraclass correlation coefficient with a power (1-β) of 0.80 and a type 1 error (α) of 0.05 were detected.
The study was approved by the center's Ethics and Clinical Trials Committee. Patients were given written information on the characteristics of the study and signed their consent to participate in it.
Conventional PSG
Conventional PSG was performed using the Siesta system (Compumedics Limited, Abbotsford, Victoria, Australia) which has 32 channels and includes an electroencephalogram (C3-A2, C4-A1), 2 channels of electro-oculogram, submental and tibial electromyogram, and measures heart rate by continuous electrocardiogram (modified V2), air flow (oral-nasal thermistor and nasal channel), chest and/or abdominal movements by piezoelectric belts, transcutaneous oxyhemoglobin saturation, body position and snoring (with a microphone). Preparation and set-up of the PSG took 45 minutes.
RPSD
The BREAS SC-20 is a level 3 RPSD,17 not long on the market, and validation studies on it have not yet been published. The system can be attended (on line) or unattended and for this study it was unattended. The system can register, store, and analyze the following signals: respiratory air flow by nasal cannula, snoring, respiratory effort by chest-wall and abdominal movements with piezoelectric belts, transcutaneous oxygen saturation and pulse by pulse oximetry, body position, ambient light, and limb movements. All channels are connected to the same device, which weighs approximately 300 g and works on rechargeable batteries. A personal computer is needed to start the test. Programming and preparation and set-up with the patient take about 15 minutes. The software of the device automatically analyzes the information as it is recorded. The recording can be manually adjusted by the technician, the corrections being included in the final report.
Methods
Overnight PSG and RPSD tests were performed on all patients, from 11:30 pm to 7:00 am, in the sleep laboratory of the hospital. PSG sensors were set up first, to ensure accurate diagnosis. Both systems were programmed to start and to finish at the same time. In order to ensure that the signal from the nasal cannula would be identical for the PSG and the RPSD, a Y-shaped cannula was devised that branched to the two devices, sending identical signals to each one. Over the following days results were stored and analyzed by different researchers highly experienced in conventional PSG and in several RPSD systems. The analysis was made independently and blind to the results of the other method. The test was considered valid after recording 300 minutes or more for the RPSD and 180 minutes of sleep for the PSG. Time taken over the analysis of the PSG was around 120 minutes; automatic RPSD, 3 to 5 minutes, and manual RPSD, 30 minutes.
Measurements
Sleep/wake PSG staging was done in 30-second intervals following Rechtschaffen and Kales' standard criteria.19 Apnea was defined as complete or almost complete (≥90%) suspension of the thermistor and/or nasal cannula signal for 10 seconds or more. Hypopnea was defined as a discernible reduction (≥50%) in the thermistor and/or nasal cannula signal accompanied by oxyhemoglobin desaturation of 3% or more and/or an electroencephalographic arousal, defined according to the American Sleep Disorders Association criteria.20 Respiratory events were classified as obstructive if they were accompanied by thoracoabdominal effort; central if there was no effort, and mixed if both occurred, normally starting with the central. AHI was defined as the sum of all the respiratory events (apneas and hypopneas) divided by the total number of hours of sleep.
Apneas and hypopneas in RPSD were classified using the same criteria as for PSG, except in the case of arousals. The respiratory events index (REI) of the RPSD was defined as the sum of respiratory events that resulted from the manual analysis divided by the total number of hours registered.
Statistical Analysis
Statistical analysis was carried out using the statistical program SPSS (version 11.0, SPSS Inc., 2001, Chicago, USA). The resultant scores were expressed as means (SD) or percentages, depending on the nature of the variable. Agreement between the AHI obtained from the PSG and the automatic or manual REI obtained from the RPSD was measured following the method described by Bland and Altman.21 The standardized difference between the AHI obtained from the PSG and the REI from the RPSD (manual or automatic) was plotted on the y-axis and the standardized mean of those values was plotted on the x-axis. The limits of agreement between the different methods were calculated using the standardized mean difference ±1.96 times the SD. The intraclass correlation coefficient was also calculated using the simplified method.22 The diagnostic validity of the results obtained from the RPSD was calculated for different AHI cut points from the PSG (≥5, ≥10, ≥15, ≥20, and ≥30). The corresponding receiver operating characteristics (ROC) curves were obtained. The diagnostic properties of the test were determined: sensitivity, specificity, positive and negative predictive values and their corresponding 95% confidence intervals, as well as the cut points that optimize sensitivity and specificity of REI obtained from the RPSD.
Results
Out of the 70 patients studied, 10 (14%) were excluded: 2 from inadequate PSG simultaneous recording; 4 from poor quality signals from the nasal cannula to the RPSD which prevented correct evaluation of all channels; 3 from not achieving 180 minutes of total sleep time, and 1 because of a technical fault at the moment the information was received. Data from 60 subjects were considered valid and are presented in Table 1.
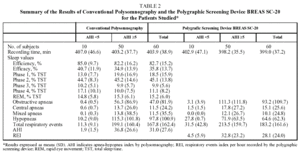
The results of the PSG and the RPSD according to the method of analysis are shown in Table 2. The mean AHI of the PSG was 31.0 (27.6) while the mean REI of the RPSD was 28.1 (24.0). The reliability of manual scoring of REI was 0.92 with respect to the AHI obtained from the PSG and the reliability of automatic scoring of REI was 0.86, according to the intraclass correlation coefficient.
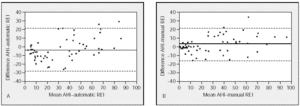
The comparison of the results of the REI obtained by automatic analysis of RPSD with those of the PSG are shown in Figure 1A. The overall mean of the differences between the AHI and the automatic REI was -3.36 (12.7). The comparison of the results of the REI obtained by manual analysis with those of the PSG are shown in Figure 1B. The overall mean of the differences between the AHI and the manual REI was 2.92 (9.75).
Figure 1. Individual differences between the apnea-hypopnea index (AHI) obtained by polysomnography and the respiratory events index (REI) by the polygraphic respiratory screening device using automatic analysis (Figure 1A) and manual analysis (Figure 1B). The continuous line (--) indicates the mean and the discontinuous lines (---) mark the limits of agreement (SD±1.96).
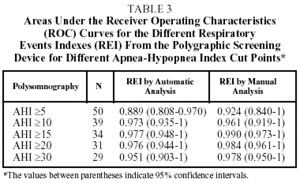
The areas under the ROC curves for several AHI cut points obtained by PSG are shown in Table 3. For an AHI cut point of ≥5, the area under the ROC curve was 0.889 for automatic analysis of RPSD, and 0.924 for manual analysis. The best results by areas under the ROC curves were obtained at the cut point ≥15.
The characteristics of the 2 methods of analysis used with the BREAS SC-20 RPSD system, corresponding to the optimum cut points for sensitivity and specificity using several SAHS severity criteria, are shown in Table 4. This table also shows the analysis of agreement between the 2 tests according to the different cut points.
Discussion
The results of our study show that the BREAS SC-20 is a highly valid RPSD with an acceptable level of accuracy for the diagnosis of patients clinically suspected of SAHS.
Most scientific associations currently consider PSG to be the standard method in the diagnosis of SAHS.12,13 For years, however, it has been suggested that in many cases PSG is not necessary23 and in some cases could even be insufficient.24 Furthermore, most experts agree that it is a complicated method, highly instrumentalized, and one that needs to be administered by a trained technician. Analysis of the results can also be difficult and time-consuming. Then again, many centers do not have PSGs readily available and there are unacceptably long waiting lists.16
Moreover, considering the high prevalence of SAHS and that unfortunately barely 5% of potential sufferers are currently diagnosed,25 we clearly need to develop simpler and cheaper diagnostic methods than the PSG, the RPSD being the most widely accepted alternative.17,27 RPSDs are cheaper than PSGs, they are easy to operate which reduces the time needed for scoring, they can be attended or unattended during the test, and can be used in outpatient facilities. This makes diagnosis in less specialized centers possible, allowing more diagnoses to be made. However, given the large number of RPSDs designed and produced to this end, the scarcity of validation studies on the systems is surprising. Technological advances occur so quickly that systems that have been validated are replaced by new models that are no longer so.
A recent systematic review on SAHS diagnosis11 examined 25 RPSDs, finding that sensitivity varied from 32% to 100%, and specificity from 33% to 100%. Differences between the diagnostic capacities of the systems, the variables they measure, and the characteristics of the populations the systems have been validated in, mean that validation results cannot be generalized to all RPSDs nor to populations with varying prevalence. Neither can results be generalized between studies carried out in laboratories and others in homes. The RPSD chosen should be appropriately validated in a similar population. Several validity studies comparing PSG with attended and unattended RPSDs have been carried out in Spain,28-34 and have shown RPSDs to be a sound diagnostic option.
We carried out a validation study on the BREAS SC-20 system, a level 3 RPSD, comparing it with PSG, in a hospital setting and unattended. The study, like others of its kind, showed a tendency to overestimate mild cases and to slightly underestimate the most severe ones. In the group of patients with an AHI >5, the mean AHI was 1.9 (1.5), whereas the REI was 4.5 (5.9). The overestimation of REI could be due to the fact that time asleep is not known and doubtful events with the subject awake tend to be counted. On the other hand, REI is divided by the number of recording hours not the number of hours of sleep, which causes the REI to be lower than the AHI (28.1 [24.0] compared with 31.0 [27.6]). Analysis of the type of events showed that the RPSD tended to underestimate hypopneas (97.8 [100.9] for the PSG compared with 64.6 [62.3] for the RPSD) and to overestimate the apneas (47.0 [81.9] for the PSG compared with 93.2 [109.7] for the RPSD). This could be due to air escaping from the mouth during the test, resulting in hypopneas being counted as apneas, whereas hypopneas are easier to detect with the PSG, which has both nasal cannula and thermistor. However, the difference between apneas and hypopneas, apart from being a complex definition,35 is of epidemiological interest but not of great clinical importance.9 Manual analysis gave better results than automatic analysis, as it has in other studies,17,28-34 though the good results achieved with automatic analysis in this system made the manual analysis easier and quicker.
In practical terms, if the RPSD is considered as a test for ruling out SAHS, (AHI <5 by PSG) the optimum cut point for the REI is 3.6 with a sensitivity of 98% (95% confidence interval 94-100). This means that the likelihood of disease is low for a patient suspected of SAHS based on clinical picture and with a REI >3.6 (a 12% posttest probability of disease given a negative test). On the other hand, if RPSD is considered as a test for confirming a diagnosis of severe SAHS (AHI ≥30 by PSG), a patient suspected of SAHS based on clinical picture who has a REI ≥31 will very likely have the syndrome, with a specificity of 100% and a 100% posttest probability given a positive test.
In order to carry out a more detailed analysis, we studied the number of patients correctly classified by the system, with scores of 83% to 95% for the various AHI cut points and high x values. RPSD validity was better for high AHI scores. Discrepancy in the classification could occur with low AHI scores, corresponding to patients with fewer therapeutical implications. Revision of the patients incorrectly classified showed that only one was a false negative, with a REI score of 0.31 and an AHI by PSG of 5.2, very close to the critical score defining SAHS, and one that would not have probably changed the therapeutic decision. On the other hand, 2 REI scores by RPSD that confirmed the patient as having SAHS (9.3 and 19.5) were contradicted by PSG with AHI scores of 1.2 and 4.0 respectively. However, neither case would have been classified as severe by RPSD. When results were analyzed considering severity criteria--AHI (PSG) ≥30 and REI (RPSD) ≥3--6 false negatives became apparent; AHI values by PSG were 31.8, 34.4, 37.2, 41.4, 44.6, and 50.8 corresponding to REI scores of 18.8, 17.2, 25.1, 28.1, 27.6, and 28.4. These patients had sleep efficiency (sleep time/recording time in minutes x 100) of 89.6%, 83.4%, 70.1%, 70.6%, 96.1%, and 73% respectively. For this reason, in cases 3, 4, and 6, low sleep efficiency could at least partly explain the differences between the PSG and the RPSD as well as the tendency of the RPSD to overestimate the severity of these cases. In addition, patient 2 had cardiovascular comorbidity and patients 3, 4, and 6 had concomitant cardiovascular and respiratory disease. The comorbidity variable can make RPSD scoring more difficult. In patients 1 and 5 there was no apparent reason for the discrepancy except for limitation in the RPSD method; nevertheless, it must be pointed out that all 6 patients would have been diagnosed as having SAHS, it was only the degree that was inaccurate, being moderate instead of severe.
The reduced number of subjects was a limitation of the study, a larger sample may modify the results. However, estimation of the sample size using the intraclass correlation coefficient suggests the sample size was sufficient. The results were consistent throughout the analysis, another factor that suggests that a larger sample size would not have made any relevant difference to the results. Another aspect to mention is the high prevalence of SAHS in the sample, and that the study, despite being unattended, was carried out in hospital. Applying these data to a population of different characteristics or using different methods could produce different results. The fact that the PSG and the RPSD were recorded simultaneously prevented any intraindividual variability that would have reduced internal validity. A strength of the study was that the main respiratory flow recording was identical for both methods (PSG and RPSD), as the signal came through the Y-shaped nasal cannula.
In summary, the BREAS SC-20 RPSD is a valid system for the diagnosis of patients clinically suspected of SAHS. Manual analysis allows patients to be classified according to whether or not SAHS is present and provides a severity index. This RPSD is therefore a good alternative to PSG in the diagnosis of SAHS. However patients with unclear results or who have a high clinical indication of SAHS with a normal or nearly normal RPSD score should consider having a PSG.
Acknowledgments
We would like to thank Ms Carmen Téllez for her help in enrolling patients; our sleep clinic nurse, Elena Leuza, for her kindness and help in preparing the systems, and Breas Medical for supplying us with the devices to carry out the study.
Correspondence: Dr. J. Durán-Cantolla.
Unidad del Sueño. Servicio de Neumología. Hospital Txagorritxu.
José Achotegui, s/n. 01009 Vitoria. Álava. España.
E-mail: joaquin.duran@wanadoo.es
Manuscript received April 4, 2003. Accepted for publication, June 16, 2003.