The ALAT and SEPAR Treatment and Control of Smoking Groups have collaborated in the preparation of this document which attempts to answer, by way of PICO methodology, different questions on health interventions for helping COPD patients to stop smoking.
The main recommendations are: (i) moderate-quality evidence and strong recommendation for performing spirometry in COPD patients and in smokers with a high risk of developing the disease, as a motivational tool (particularly for showing evidence of lung age), a diagnostic tool, and for active case-finding; (ii) high-quality evidence and strong recommendation for using intensive dedicated behavioral counseling and drug treatment for helping COPD patients to stop smoking; (iii) high-quality evidence and strong recommendation for initiating interventions for helping COPD patients to stop smoking during hospitalization with improvement when the intervention is prolonged after discharge, and (iv) high-quality evidence and strong recommendation for funding treatment of smoking in COPD patients, in view of the impact on health and health economics.
Los grupos de control y tratamiento del tabaquismo de ALAT y SEPAR han colaborado para la realización de este documento en el que se da respuesta, siguiendo metodología PICO, a diferentes interrogantes relacionados con la asistencia sanitaria para ayudar a dejar de fumar a los pacientes con EPOC.
Sus principales recomendaciones son: a) evidencia moderada y recomendación fuerte para realizar espirometría en pacientes con diagnóstico o en fumadores con alto riesgo de padecer EPOC, como instrumento de motivación, en particular evidenciando la edad pulmonar, y con fines diagnósticos y de búsqueda activa de casos; b) evidencia alta y recomendación fuerte para utilizar asesoramiento conductual intenso y específico y tratamiento farmacológico para ayudar a dejar de fumar a fumadores con EPOC; c) evidencia alta y recomendación fuerte para iniciar intervenciones para ayudar a dejar de fumar a fumadores con EPOC mientras se encuentran hospitalizados mejorando al mantener la intervención tras el alta, y d) evidencia alta y recomendación fuerte para la financiación del tratamiento del tabaquismo en fumadores con EPOC por su impacto sobre la salud y la economía de la salud.
Approximately 85% of chronic obstructive pulmonary disease (COPD) is caused by tobacco consumption. Smoking cessation is the only measure that has been shown to be effective for halting the progression of the disease.1 Various papers have analyzed the characteristics of health interventions for smoking cessation in smokers with COPD.2–6 In all of these, some outstanding questions remain to be answered using scientific methodology.
The Treatment and Control of Smoking Groups of the Latin-American Thoracic Association (ALAT) and the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) have collaborated in the preparation of this document which uses PICO methodology to answer questions on smoking cessation treatment in COPD patients. The main objective of this document is to provide healthcare professionals with up-to-date scientific information in this area.
MethodologyA collaborative group was formed and clinical questions were formulated. Four working subgroups were constituted, formed of members of the two medical associations involved, ALAT and SEPAR. These subgroups drew up a list of clinical questions and selected by consensus those that should be addressed in these recommendations. In order to focus the search for available evidence, all clinical questions were transformed to the PICO format or the PECO variant: Patient (problem or population), Intervention or Exposure, Comparison, and Outcome.7
The literature search strategy was conducted simultaneously in 2 metasearch engines, the Trip database and PubMed, using MeSH (Table 1).
Search Strategy (Trip Database Key Words and MeSH Terms).
| Clinical Question | PICO Question | Search Strategy Using MeSH Terms |
|---|---|---|
| Does spirometry motivate the smoker with COPD to stop smoking? | (a) P=COPD or Chronic Obstructive Pulmonary Disease I=Smoking Cessation C=Ø O=Lung age | ((((“pulmonary disease, chronic obstructive” [MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction))) AND “Smoking Cessation” [MeSH]) AND lung age |
| (b) P=COPD or Chronic Obstructive Pulmonary Disease I=Smoking Cessation C=Ø O=(“Spirometry” [MeSH] OR Spirometries) | ((((((“pulmonary disease, chronic obstructive” [MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction))))) AND ((cessation, smoking OR cessations, smoking OR smoking cessation) OR “Smoking Cessation” [MeSH])) AND (“Spirometry” [MeSH] OR Spirometries) | |
| Is the treatment of smoking different between smokers with and without COPD? | (a) P=COPD or Chronic Obstructive Pulmonary Disease I=Smoking Cessation C=Ø O=Ø | (((((“pulmonary disease, chronic obstructive” [MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction))))) AND ((cessation, smoking OR cessations, smoking OR smoking cessation) OR “Smoking Cessation” [MeSH]) by relevance |
| (b) P=Smokers with COPD versus Smokers without COPD I=Smoking cessation treatment C=Ø O=Ø | ||
| Is smoking cessation intervention effective in hospitalized smokers with COPD? | (a) P=COPD or Chronic Obstructive Pulmonary Disease I=Smoking Cessation C=Ø O=Hospitalization | (((((((“pulmonary disease, chronic obstructive” [MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction))))) AND ((cessation, smoking OR cessations, smoking OR smoking cessation) OR “Smoking Cessation” [MeSH]))) AND (“Hospitalization” [MeSH] OR hospitalizations OR hospitalized) Sort by: Relevance |
| (b) P=COPD or Chronic Obstructive Pulmonary Disease I=Smoking Cessation C=Ø O=Exacerbation | ||
| Would funding the treatment of smoking in smokers with COPD impact on health economics? | P=COPD or Chronic Obstructive Pulmonary Disease I=Smoking Cessation C=Ø O=financing OR price OR pay OR incentives | ((((((“pulmonary disease, chronic obstructive” [MeSH Terms] OR COPD OR Chronic Obstructive Pulmonary Disease OR COAD OR Chronic Obstructive Airway Disease OR Chronic Obstructive Lung Disease OR Airflow Obstruction, Chronic OR Airflow Obstructions, Chronic OR Chronic Airflow Obstructions OR Chronic Airflow Obstruction))))) AND ((cessation, smoking OR cessations, smoking OR smoking cessation) OR “Smoking Cessation” [MeSH])) AND (financing OR price OR pay OR incentives) |
The results retrieved for PICO questions were prioritized according to the highest level of evidence (randomized controlled trials [RCTs], meta-analyses and systematic reviews) and the most appropriate answer to the clinical question. If this was not possible, intermediate (observational) or low level (open-label, case series or consensus) studies in the hierarchy of evidence were selected. The recommended algorithmic selection method was used primarily for therapeutic questions.8 Studies published in Spanish, Portuguese and English were considered for inclusion. The end date of the search was May 2016 (Table 2).
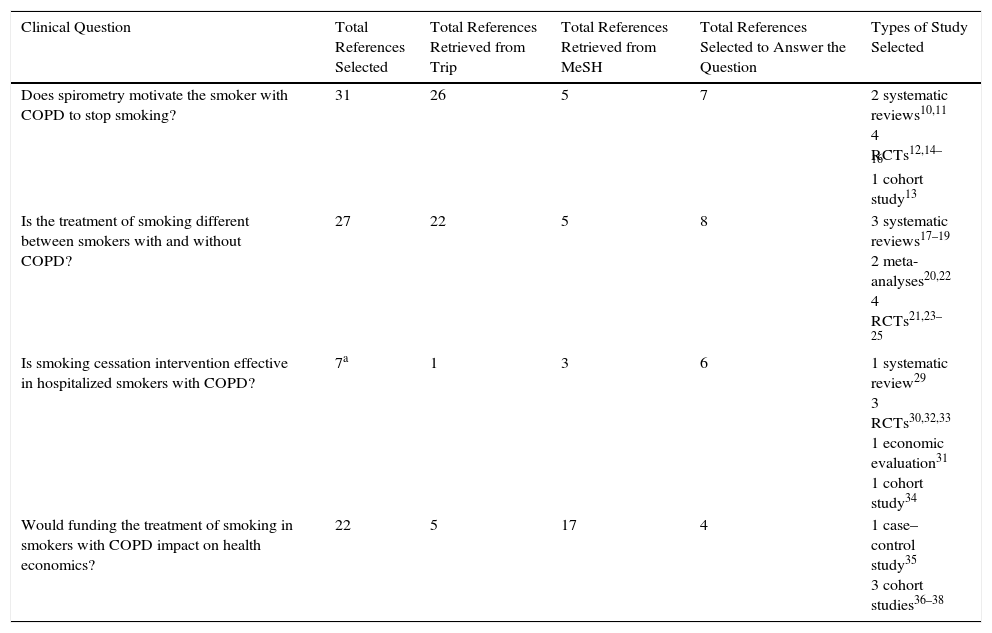
Number and Type of Studies Selected to Answer the Clinical Questions.
| Clinical Question | Total References Selected | Total References Retrieved from Trip | Total References Retrieved from MeSH | Total References Selected to Answer the Question | Types of Study Selected |
|---|---|---|---|---|---|
| Does spirometry motivate the smoker with COPD to stop smoking? | 31 | 26 | 5 | 7 | 2 systematic reviews10,11 4 RCTs12,14–16 1 cohort study13 |
| Is the treatment of smoking different between smokers with and without COPD? | 27 | 22 | 5 | 8 | 3 systematic reviews17–19 2 meta-analyses20,22 4 RCTs21,23–25 |
| Is smoking cessation intervention effective in hospitalized smokers with COPD? | 7a | 1 | 3 | 6 | 1 systematic review29 3 RCTs30,32,33 1 economic evaluation31 1 cohort study34 |
| Would funding the treatment of smoking in smokers with COPD impact on health economics? | 22 | 5 | 17 | 4 | 1 case–control study35 3 cohort studies36–38 |
Recommendations and templates developed by the CASPE network (www.redcaspe.org) were used for the critical appraisal of the selected references. The ACCP grading system was used to classify recommendations as strong1 or weak,2 according to the balance of risk, benefits, burdens, and, in some cases, cost. The quality of evidence was classified as high (A), moderate (B) or low (C), depending on the study design, consistency of results, and ability of the evidence to clearly answer the PICO question.9
The authors appointed two external reviewers with expertise in the field of COPD and smoking.
PICO QuestionDoes spirometry motivate the smoker with COPD to stop smoking?
RationaleSignificant efforts have been made to increase the rate of smoking cessation using motivational tools and measures, such as spirometry measurements, co-oximetry, and the calculation of lung age. It is of interest to determine the status of current clinical research aimed at answering this question.
Search ResultsThirty-one references were retrieved (MeSH: 5; Trip database: 26), and 7 studies were selected to answer the question (2 systematic reviews, 4 randomized clinical trials [RCT], and 1 cohort study).
Quality of EvidenceOne systematic review found limited benefits from the use of spirometry as a motivating factor for increasing smoking cessation,10 and another11 evaluated the effectiveness of the use of biomedical instruments for increasing motivation in smoking cessation. Both included 15 RCTs, 2 of which used spirometry in primary care as a motivator. No statistically significant benefit was identified in the smoking cessation rate when spirometry was used. The analysis does not evaluate the motivating effect of the spirometry test when the result was abnormal. In this respect, a more recent RCT12 showed that bronchial obstruction was the most significant predictor of smoking cessation (OR: 4.215; 95% CI: 2.215–7.865). Similarly, in another study,13 4494 smokers selected from 100000 subjects with obstructive spirometry were informed in a phone call about COPD and given brief smoking cessation counseling. The results showed a cessation rate of 16.3% among patients with obstruction compared to 12% among those with normal spirometry (P=.0003). An RCT14 which compared COPD patients to smokers with normal spirometry, and included a 3-year follow-up and pharmacological interventions, found a higher rate of cessation among smokers with COPD at the 3-year follow-up (29% versus 14%; P=.003).
As for the motivating effect of explaining to the patient their lung age calculated according to spirometry, an RCT15 found a smoking cessation rate at 12 months of 13.6% compared to 6.4% in the control group (P=.05; NNT=14).
An RCT16 failed to show efficacy when smokers with no previous diagnosis of COPD were confronted with spirometry results, even though the cessation rate was twice that of the control group (OR: 2.02; 95% CI: 0.63–6.46).
Conclusions and RecommendationsModerate evidence that spirometry results encourage smoking cessation in patients with COPD or in at-risk smokers.
Strong recommendation to perform spirometry in patients with a diagnosis or in symptomatic smokers as a motivational tool and for diagnostic purposes in active case-finding.
Grade of recommendation: 1B.
PICO QuestionIs the treatment of smoking different between smokers with and without COPD?
RationaleThe conclusions from studies analyzing the treatment of smoking among smokers with COPD suggest that the combination of psychological counseling and pharmacological treatment is most effective.2,17–19
It seems clear that when the intensity of psychological counseling is mild, efficacy in this group of smokers is very limited or non-existent. In smokers with COPD, psychological counseling should be intense both in terms of number of sessions and time dedicated to each session. Counseling must be offered with empathy, respect and understanding for the patient. The intimate relationship between the consumption of tobacco and COPD should be clearly explained to the patient, and they must be shown that the only treatment that has been identified as capable of controlling the chronic evolution of the disease is to stop smoking. Similarly, it must emphasized that in COPD, giving up smoking contributes definitively to the control of the four major symptoms of COPD: cough, expectoration, dyspnea, and chest tightness.2,17–19
Three types of drugs have been used in clinical studies: nicotine replacement therapy (NRT), varenicline and bupropion. All these studies have shown that the association between active drug and psychological counseling is more effective than the association between counseling and placebo. NRT and varenicline have been shown to be effective at 6 and 12 months of follow-up, whereas bupropion was effective at 6 months only.2,17–19
A study has found that the efficacy rates in these patients are higher when pharmacological treatment is offered in addition to counseling (for more than 3 months, or in a combination of several drugs, or at high doses).3,4
It is important to determine which components are indispensable in the counseling of smokers, in order to make it more effective. It is also important to determine how to provide the most effective pharmacological treatment.
Search SelectionThirty-one references were retrieved (MeSH: 5; Trip database: 22), and 8 studies were selected to answer the question (3 systematic reviews, 2 meta-analyses, 2 RCTs, and 1 cross-sectional study).
Summary of EvidenceOne of the first reviews on smoking cessation in smokers with COPD showed that the combination of behavioral counseling and pharmacological treatment was superior to no treatment (RR: 4.0; 95% CI: 3.25–4.93) or the use of behavioral counseling only (RR: 4.19; 95% CI: 3.41–5.15).17
Another meta-analysis evaluated 7332 patients with COPD who received different treatments for smoking cessation, and found that the combination of behavioral counseling plus NRT was the most effective type of intervention (OR: 5.08; P<.0001) compared to behavioral counseling only (OR: 2.8; P=.001) and compared to behavioral counseling in combination with an antidepressant (OR: 3.32; P=.002). Behavioral counseling only came close to achieving efficacy when compared with standard care (OR: 1.81; P=.07).20
Another study evaluated the effectiveness of high-intensity smoking treatment in smokers with COPD compared with standard care. The high-intensity program consisted of a combination of pharmacological treatment plus hospitalization for 2 weeks and the administration of intensive behavioral counseling; telephone contacts and continuous monitoring were also programmed for a period of 1–3 years. Abstinence figures in the group that received the intensive treatment were 52% and 38% at 1 and 3 years of follow-up, respectively, vs 7% and 10%, respectively, in the group that received standard care.21
One of studies that has shed most light on the issue is probably that of Hoogendoon et al.,18 who conducted a systematic review of various clinical trials in patients with COPD. The different interventions were grouped into 4 categories: standard care, minimal intervention, intensive behavioral counseling, and intensive behavioral counseling plus pharmacological treatment. The abstinence rates after 1 year of follow-up for each of the categories were: 1.4%, 2.6%, 6% and 12.3%, respectively. Compared to standard treatment, the cost per quality-adjusted life year (QALY) gained for the minimum intervention was 16900 euros, 8200 euros for intensive behavioral counseling, and 2400 euros for intensive behavioral counseling plus pharmacological treatment. The authors conclude that the combination of intensive behavioral counseling plus pharmacological treatment is the most successful and cost-effective treatment of smoking in COPD patients.
Bartlett et al.22 evaluated 17 randomized trials to determine what type of behavioral counseling for smoking cessation is most effective in smokers with COPD. This study identified 5 counseling techniques that are significantly associated with the best results: (1) action planning; (2) recording course of symptoms, achievements, and failures; (3) weight control; (4) social support; and (5) explaining clearly explain the relationship between COPD and smoking.
Two clinical trials have examined the efficacy and safety of bupropion. One found that bupropion was more effective than placebo in achieving continuous abstinence at 6 months of follow-up (16% vs 9%; P<.05).23 The other also showed similar results at 6 months of follow-up (difference of 18.9% [95% CI: 3.6–34.2]; P=.02.24
The efficacy and safety of varenicline was analyzed in an RCT which found that varenicline was more effective than placebo for helping smokers to quit at 3, 6 and 12 months of follow-up. At the end of the year, the figures were 18.6% vs 5.6% (OR: 4.04 [95% CI: 2.13–7.67]; P<.0001).25
A recent review analyzing 16 studies that included more than 13000 patients has reached similar conclusions to those discussed here.26
Conclusions and RecommendationsThe available evidence indicates that the most effective intervention to help subjects with COPD to quit smoking is a combination of intensive behavioral counseling plus pharmacological treatment. This intervention is highly cost-effective.
Behavioral counseling should be intensive and prolonged; the greater the intensity and the longer the duration, the greater the efficacy.
A choice of NRT, bupropion or varenicline can be used as pharmacological treatment. Bupropion showed efficacy only at 6 months of follow-up. NRT and varenicline have been shown to be effective at 6 and 12 months of follow-up.
High evidence and strong recommendation for intensive behavioral counseling plus pharmacological treatment (NRT and varenicline are superior to bupropion) to help smokers with COPD to quit.
Grade of recommendation: 1A.
PICO QuestionIs smoking cessation intervention effective in hospitalized smokers with COPD?
RationaleAround 25%–35% of COPD patients admitted to hospital are smokers. Hospitalization is an ideal situation to help smokers quit. Taking into account these premises, hospitalization of an individual with COPD represents an important opportunity to determine their smoking status and to intervene decisively.27,28
Search SelectionSeven references were retrieved (MeSH: 3; Trip database: 1; and by snow-balling: 3), and 6 studies were selected to answer the question (3 RCTs, 1 systematic review, 1 economic evaluation, and 1 cohort study).
Summary of EvidenceA systematic review found that intensive behavioral interventions that begin in the hospital and continue for at least 1 month after discharge increase abstinence rates (RR: 1.37; 95% CI: 1.27–1.48).29 One study found that the combination of intensive intervention plus NRT increased abstinence rates compared with intensive intervention alone (RR: 1.54; 95% CI: 1.34–1.79). However, 2 other studies failed to show that adding varenicline or bupropion increases abstinence rates. Similar results were found in a subgroup of smokers who were admitted for cardiovascular diseases; in this subgroup, intensive intervention followed by subsequent support increased abstinence rates (RR: 1.42; 95% CI: 1.29–1.56) when compared with non-intensive intervention.
Abstinence rates were compared in 2 groups of smokers enrolled in a clinical trial.30 One group received intensive intervention (free pharmacological treatment for 90 days, regular phone calls and behavioral reinforcement), while the control group only received recommendations on the use of the medication at the time of discharge. Point prevalence abstinence at 6 months was significantly higher in the intervention group (26% vs 15%; RR: 1.71; 95% CI: 1.14–2.56; P=.009; NNT: 9.4; 95% CI: 6.4–35.5). The same was observed for continuous abstinence rates at 6 months (27% vs 16%; RR: 1.70; 95% CI: 1.15–2.51).
A pharmacoeconomic study analyzed the cost-effectiveness of the Ottawa model for smoking cessation (OMSC) in patients hospitalized for various diseases, including COPD.31 The OMSC includes psychological counseling and pharmacological treatment. The authors found that the OMSC program was very cost-effective with a 1-year cost per QALY gained of C$1386, and lifetime cost per QALY gained of C$68. The study showed that using the Ottawa model in 15326 hospitalized smokers in 1 year would achieve a total of 4689 quitters, and would prevent 116 re-hospitalizations, 923 hospital days, and 119 deaths in all of these chronic diseases.
A clinical trial carried out by Borglykke et al.32 compared the rates of abstinence in 2 groups of smokers with COPD attending the emergency department of a hospital. One group was randomized to receive intensive intervention and the other was the control group. Patients were followed up at 1 year and 3 years after the intervention. The results showed that at the 1-year follow-up, the abstinence rate in the intervention group was significantly higher than in the control group (30% vs 13%; OR: 2.83; 95% CI: 1.40–5.74). Moreover, no significant differences were found in terms of the amount of phlegm or survival (50.4% vs 43.1%; HR: 0.80; 95% CI: 0.55–1.16). However, at the 3-year follow-up, the intervention group had been hospitalized significantly fewer days for COPD or other causes.
A clinical trial33 randomized 172 patients to 2 groups. The intervention group received medical counseling for quitting smoking and screening for gastroesophageal reflux, depression and/or anxiety, in addition to education on the use of inhalers. Compared with the control group, the results showed no significant differences in readmissions or consultations within 30 days after discharge, or in the rate of readmissions at 90 days.
Another study in a cohort of COPD patients hospitalized for exacerbations34 reviewed the effectiveness of a brief anti-smoking counseling session during admission and the use of medication to assist smoking cessation after discharge. A total of 1334 patients were included: 19.8% reported having stopped smoking at 6 and 12 months of follow-up, 17.5% had no reported status, and 63.7% continued smoking. It is interesting to note that only 33.7% of the patients used smoking cessation medication. The results did not show higher rates of abstinence among those who received medication to quit smoking. Nor was any association found between anti-smoking counseling at the time of discharge and abstinence, or between those who received medication in the 48-h period after discharge or in those who had received treatment in the year prior to admission. Patients who received varenicline presented significantly higher rates of abstinence (OR: 2.44; 95% CI: 1.48–4.05), while those who received NRT in monotherapy showed the worst results (OR: 0.66; 95% CI: 0.51–0.85). Patients treated with bupropion or combination therapy were no more likely to report cessation.
Conclusions and RecommendationsThe available evidence indicates that intensive intervention to help quit smoking initiated while the patient is hospitalized plus at least 1 month of follow-up after hospital discharge achieves higher abstinence rates when compared with smokers who received standard treatment. Effectiveness increases significantly when psychological counseling is added to treatment with NRT or varenicline. This intervention is highly cost-effective.
High evidence and strong recommendation for initiating interventions to help COPD patients stop smoking during hospitalization.
Grade of recommendation: 1A.
PICO QuestionWould funding the treatment of smoking in smokers with COPD impact on health economics?
RationaleCOPD places a considerable economic burden on public health systems. In Spain, costs amount to €3bn. a year. It is also known that the cost of care incurred by a smoker with COPD is approximately €3700/year, while individuals with COPD who have stopped smoking incur costs of €2000/year. Accordingly, then, when a smoker with COPD quits, savings to the National Health System amount to around €1300/year.35
Search SelectionTwenty-two references were retrieved (MeSH: 17; Trip database: 5), and 4 studies were selected to answer the question (3 cohort studies and 1 case–control).
Summary of EvidenceA case–control study conducted in Spain, in which costs generated by smokers with COPD were compared with those of former smokers with COPD over a 12-month period, found that the smokers with COPD consumed more health care resources compared to former smokers, regardless of their COPD severity stage.35
Three simulated cohort studies in the United Kingdom, Spain and France have analyzed the scenario of funding smoking cessation in patients with COPD using a Markov model: (1) in COPD patients in the United Kingdom,36 the ICER (incremental cost-effectiveness ratio) was £2686 per QALY and continued to yield savings after 10 years in all patients, regardless of COPD severity and percentage of success of the intervention applied; (2) in Spain,37 the model was developed with a time horizon of 5 years and considered the 3 approved first-line drugs (varenicline, bupropion and NRT). Estimates were that 17756 COPD patients would give up smoking if the treatment were funded, compared to 1303 patients if it was not.38 In the reimbursement scenario, accumulated savings would be €4.3 million; (3) in France, a cost-effectiveness study in patients with COPD, cancer, and cardiovascular disease allowed the authors to conclude that for a 5-year horizon, cost savings would be made in the range of €15–215 million with an ICER of €11187 per QALY.
Conclusions and RecommendationsStudies analyzing the impact of funding of smoking cessation in patients with COPD make the following conclusions: (a) COPD patients who continue to smoke use more health care resources and generate greater social and economic costs; and (b) the funding of smoking cessation in smokers with COPD is highly effective and cost-effective.
Moderate evidence and strong recommendation for the funding of the treatment of smoking in smokers with COPD, due to the impact on health and health economics.
Grade of recommendation: 1B.
Conflict of InterestsDr. Carlos A. Jiménez Ruiz has worked with GSK and Pfizer, pharmaceutical companies with an interest in the field of the treatment of smoking.
Dr. Daniel Buljubasich has no conflict of interest.
Dr. Juan A. Riesco Miranda has worked with GSK, Pfizer, Novartis, Boehringer Ingelheim and Astra-Zeneca in training and clinical research activities.
Dr. Augustín Acuña Izcaray has received fees for lectures and/or scientific consultancy from Astra-Zeneca, Novartis and Pfizer.
Dr. José Ignacio de Granda Orive has no conflict of interest.
Dr. José Miguel Chatkin has no conflict of interest.
Dr. Gustavo Zabert received fees for educational activities from Global Bridges and the InterAmerican Heart Foundation and for speaking engagements from Astra-Zeneca, Novartis, Boehringer Ingelheim, and Pfizer.
Dr. Alfredo Guerreros Benavides has no conflict of interest.
Dr. Nelson Paez Espinel has no conflict of interest.
Mrs. Valeri Noah has no conflict of interest.
Dr. Efraín Sánchez-Angarita has received fees for lectures at conferences and educational activities related to COPD treatment from Novartis, Boehringer Ingelheim, Takeda, and Pfizer.
Dr. Ingrid Núñez-Sánchez has no conflict of interest.
Dr. Dr. Raúl H. Sansores has no conflict of interest.
Dr. Alejandro Casas has no conflict of interest.
Dr. Andres Palomar Lever has received fees for speaking engagements from Novartis and Actelion.
Dr. Inmaculada Alfageme Michavila has no conflict of interest.
We thank Dr. Ángela Ramos Pinedo (Hospital Fundación Alcorcón, Madrid, Spain) and Dr. Susana Luhning (Professor of the Department of Internal Medicine/Respiratory Medicine, Facultad de Medicina, Universidad de Córdoba, Argentina), who acted as external reviewers of this document.
Please cite this article as: Jiménez Ruiz CA, Buljubasich D, Riesco Miranda JA, Acuña Izcaray A, de Granda Orive JI, Chatkin JM, et al. Preguntas y respuestas relacionadas con tabaquismo en pacientes con EPOC. Aplicación de metodología con formato PICO. Arch Bronconeumol. 2017;53:622–628.