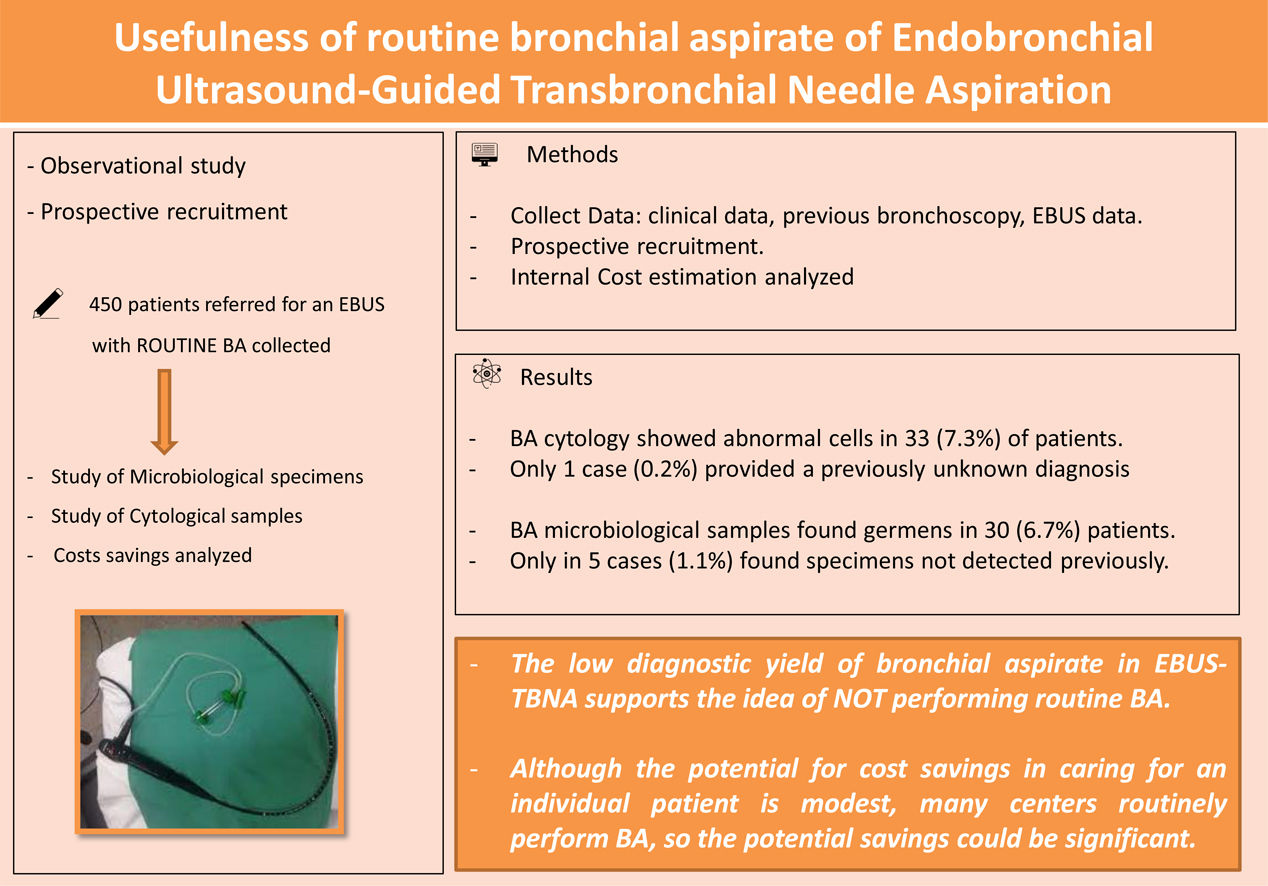
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is commonly used for the study of intrathoracic lymphadenopathy and centrally tumours but no report has discussed the contribution of routine cytological and microbiological BA during the procedure. The aim of the study was to analyse the diagnostic yield of BA during EBUS, and to determine the potential cost reduction.
MethodsA prospective study of cytological and microbiological BA collected during EBUS-TBNA was conducted between January 2021 and June 2022. Demographic data, indication, previous BA bronchoscopy or EBUS diagnosis were recorded. The main variable tested was the number of patients in which the result of the BA obtained through EBUS-TBNA determined a change in the diagnosis.
ResultsA total of 450 (70.9% male) patients were included. BA cytology showed abnormal cells in 33 (7.3%) of patients, and only 1 case (0.2%) provided a previously unknown diagnosis. All these cases were patients with suspected malignancy. BA microbiological samples found germens in 30 (6.7%) patients but only in 5 cases (1.1%) found microbiological specimens not detected in previous bronchoscopy. None of them received antibiotics and evolved correctly. The potential total cost reduction during the study period at our centre if routine BA was deleted would be 21,937.50€ for routinely combined study.
ConclusionsThe low diagnostic yield of cytological and microbiological bronchial aspirate in EBUS-TBNA supports the idea of not performing routine BA. Although the potential for cost savings in caring for an individual patient is modest, many centres routinely perform BA, so the potential savings could be significant.
Endobronchial ultrasound (EBUS) is a technology that allows real-time visualisation of structures adjacent to the airways during bronchoscopy.1 Endobronchial ultrasound transbronchial needle aspiration (EBUS-TBNA) has become the procedure of choice for a minimally invasive approach for the management of intrathoracic lymphadenopathy and lung lesions.2,3
As EBUS-TBNA became more available, questions emerged about the optimal performance of the procedure and best conditions for a maximal diagnostic yield.1 Clinical guidelines clarify aspects such as sedation, artificial airways, ultrasonographic features, needle aspects, samples and training,1,4,5 but no report has discussed the contribution of cytological examination of bronchial aspirate (BA) during EBUS.
A study analysing the diagnostic yield of cytological examinations in flexible bronchoscopy and EBUS for lung malignancies, demonstrates a low sensitivity (less than 15%) from bronchial aspirate or washing collected during bronchoscopy, suggesting avoiding the BA collection routinely.6 Despite this, BA is routinely used in most centres during EBUS.
The aim of this study was to analyse the usefulness and the diagnostic yield of BA during EBUS, and to determine the potential cost reduction.
Material and MethodsDesign and PatientsThis is a prospective study of patients submitted to an EBUS-TBNA with BA collected. Patients who underwent EBUS-TBNA for study of the mediastinum with a BA collected from January 2021 to June 2022 were included. EBUS-TBNA indications were diagnostic or staging lung cancer, or re-staging and lymphadenopathy study. All patients signed written informed consents and the study was approved by the Ethics Committee.
PerformanceLinear EBUS was performed (BFUC180 F, Olympus Optical Co Ltd., Tokyo, Japan), under local anaesthesia and sedation, using topical lidocaine spray and intravenous midazolam, propofol, and/or fentanyl in accordance with standard recommendations.7 The aspirated material was placed in two sterile taps and sent to the microbiology and cytology laboratory respectively.
Cost EstimationThe institutional cost for patients who underwent a bronchial aspirate analysis at our centre was determined from the internal and external billing price. This includes materials, time and technical and professional fees. Cytological analysis of BA costs 27.50€ and microbiological analysis 21.25€.
Statistical AnalysisThe main variable tested in our study was the number of patients in which the result of the BA obtained through EBUS-TBNA determined a change in the diagnosis.
Data were entered into a database and analysed using SPSS software (SPSS Inc. Released 2009. PASW Statistics for Windows, Version 18.0. Chicago: SPSS Inc.). Categorical variables were expressed as absolute and relative frequencies and continuous variables as means plus standard deviation (SD).
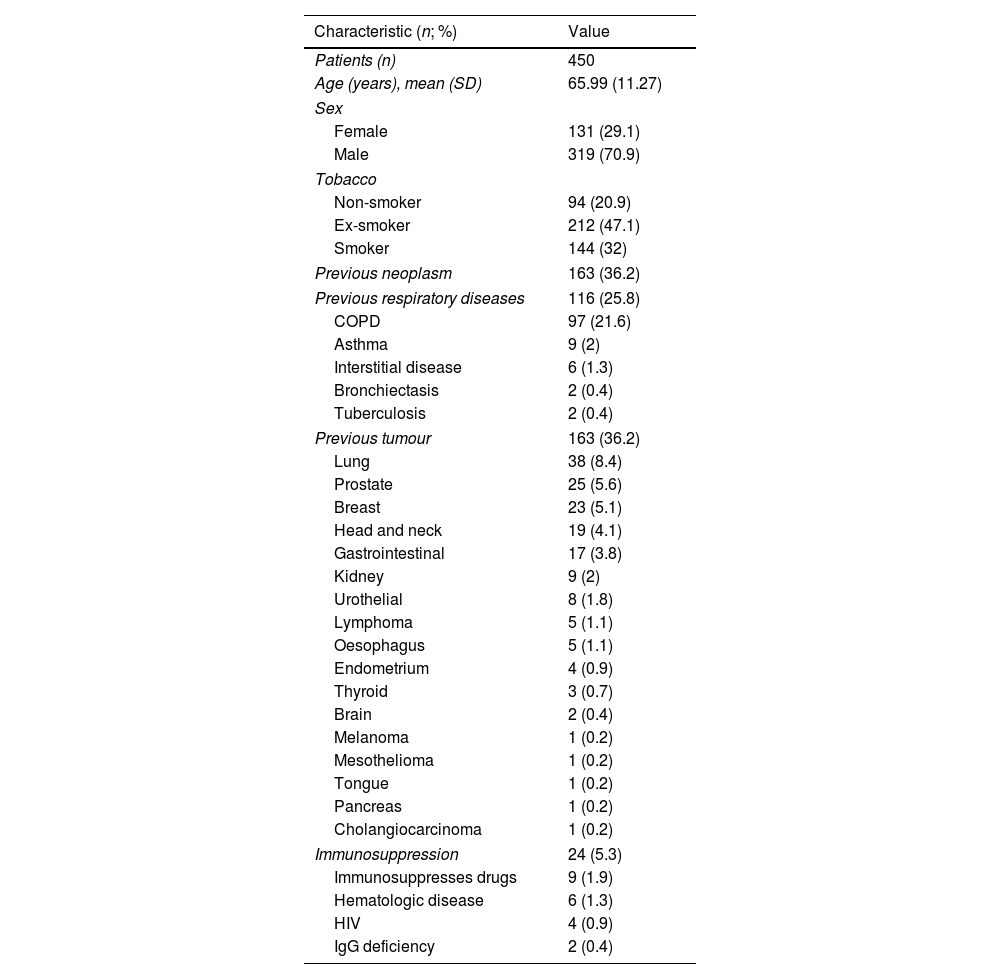
ResultsA total of 450 patients with BA collected from EBUS-TBNA were included. All BA were sent to cytology and microbiology studies. Patients’ characteristics are shown in Table 1.
Patient's Characteristics.
| Characteristic (n; %) | Value |
|---|---|
| Patients (n) | 450 |
| Age (years), mean (SD) | 65.99 (11.27) |
| Sex | |
| Female | 131 (29.1) |
| Male | 319 (70.9) |
| Tobacco | |
| Non-smoker | 94 (20.9) |
| Ex-smoker | 212 (47.1) |
| Smoker | 144 (32) |
| Previous neoplasm | 163 (36.2) |
| Previous respiratory diseases | 116 (25.8) |
| COPD | 97 (21.6) |
| Asthma | 9 (2) |
| Interstitial disease | 6 (1.3) |
| Bronchiectasis | 2 (0.4) |
| Tuberculosis | 2 (0.4) |
| Previous tumour | 163 (36.2) |
| Lung | 38 (8.4) |
| Prostate | 25 (5.6) |
| Breast | 23 (5.1) |
| Head and neck | 19 (4.1) |
| Gastrointestinal | 17 (3.8) |
| Kidney | 9 (2) |
| Urothelial | 8 (1.8) |
| Lymphoma | 5 (1.1) |
| Oesophagus | 5 (1.1) |
| Endometrium | 4 (0.9) |
| Thyroid | 3 (0.7) |
| Brain | 2 (0.4) |
| Melanoma | 1 (0.2) |
| Mesothelioma | 1 (0.2) |
| Tongue | 1 (0.2) |
| Pancreas | 1 (0.2) |
| Cholangiocarcinoma | 1 (0.2) |
| Immunosuppression | 24 (5.3) |
| Immunosuppresses drugs | 9 (1.9) |
| Hematologic disease | 6 (1.3) |
| HIV | 4 (0.9) |
| IgG deficiency | 2 (0.4) |
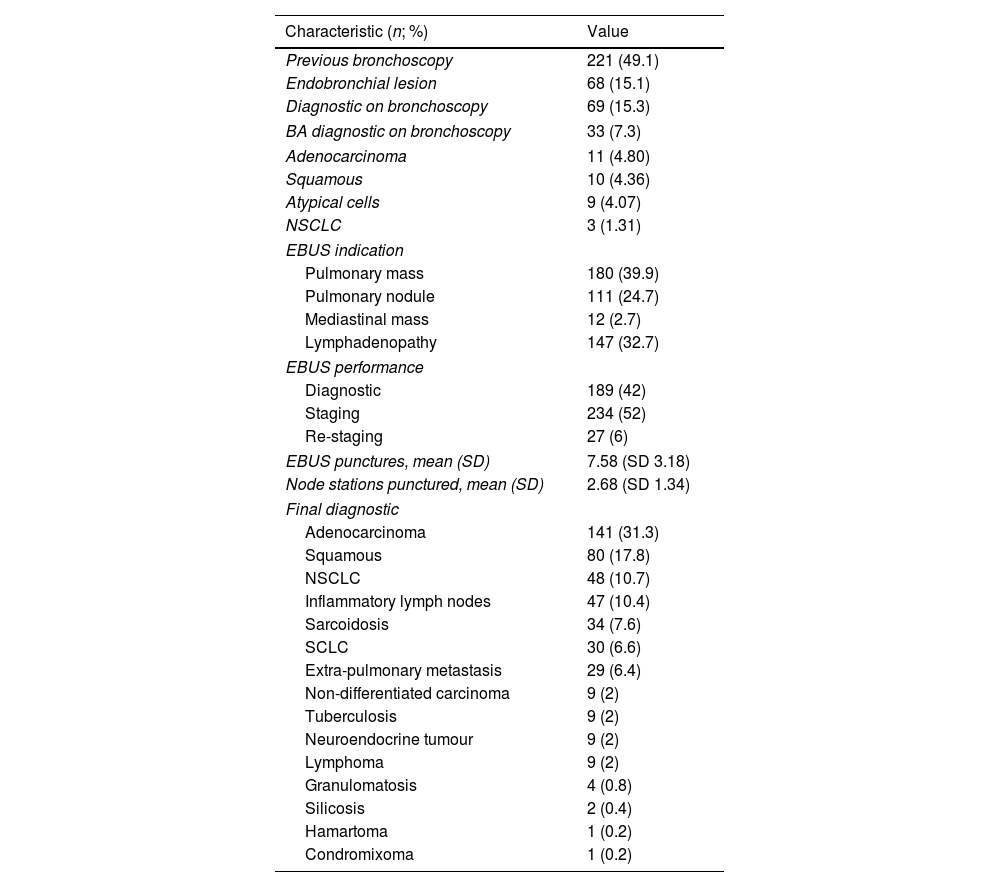
Of these, 229 (50.9%) patients have a previous bronchoscopy, and BA was diagnostic in 23 (5.1%) patients. Diagnostic or staging lung cancer was the most frequent indication. Bronchoscopy and EBUS characteristics are shown in Table 2.
Bronchoscopy and EBUS Characteristics.
| Characteristic (n; %) | Value |
|---|---|
| Previous bronchoscopy | 221 (49.1) |
| Endobronchial lesion | 68 (15.1) |
| Diagnostic on bronchoscopy | 69 (15.3) |
| BA diagnostic on bronchoscopy | 33 (7.3) |
| Adenocarcinoma | 11 (4.80) |
| Squamous | 10 (4.36) |
| Atypical cells | 9 (4.07) |
| NSCLC | 3 (1.31) |
| EBUS indication | |
| Pulmonary mass | 180 (39.9) |
| Pulmonary nodule | 111 (24.7) |
| Mediastinal mass | 12 (2.7) |
| Lymphadenopathy | 147 (32.7) |
| EBUS performance | |
| Diagnostic | 189 (42) |
| Staging | 234 (52) |
| Re-staging | 27 (6) |
| EBUS punctures, mean (SD) | 7.58 (SD 3.18) |
| Node stations punctured, mean (SD) | 2.68 (SD 1.34) |
| Final diagnostic | |
| Adenocarcinoma | 141 (31.3) |
| Squamous | 80 (17.8) |
| NSCLC | 48 (10.7) |
| Inflammatory lymph nodes | 47 (10.4) |
| Sarcoidosis | 34 (7.6) |
| SCLC | 30 (6.6) |
| Extra-pulmonary metastasis | 29 (6.4) |
| Non-differentiated carcinoma | 9 (2) |
| Tuberculosis | 9 (2) |
| Neuroendocrine tumour | 9 (2) |
| Lymphoma | 9 (2) |
| Granulomatosis | 4 (0.8) |
| Silicosis | 2 (0.4) |
| Hamartoma | 1 (0.2) |
| Condromixoma | 1 (0.2) |
EBUS-TBNA cytology was diagnostic in 232 (53.7%) of patients. Of them 209 (46.4%) showed malignant cells and 33 (7.3%) granulomas. BA cytology showed abnormal cells only in 33 (7.3%) patients, and only 1 case (0.2%) provided a previously unknown diagnosis. Of the 450 BA, 230 (51.1%) patients had a previous bronchoscopy. Of them, 21 (9.1%) BA cytology showed abnormal cells but none of them provided additional information. Two hundred and twenty (48.9%) did not have a previous bronchoscopy. Of them 12 (5.4%) BA cytology showed abnormal cells, and only 1 case (0.4%) provided a previously unknown diagnosis.
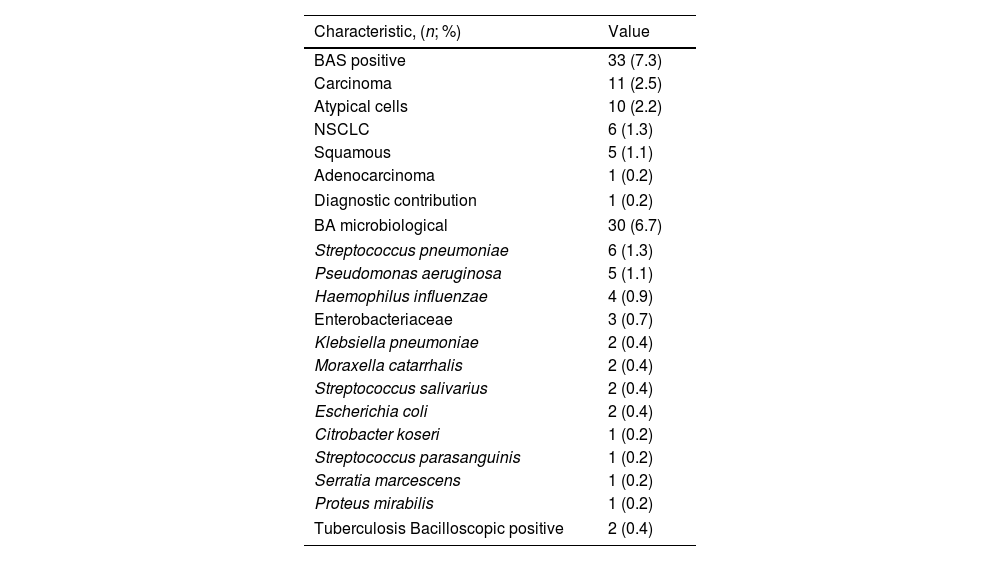
All these cases were patients with suspected malignancy: 30 cases (66.6%) of suspected lung cancer, and 3 cases (0.67%) with suspected extra-pulmonary metastasis. There were no cases of positive BA in patients with suspected benign disease, infectious disease or lymphadenopathy study without tumour. Table 3 describes cytological and microbiological results.
BA Results.
| Characteristic, (n; %) | Value |
|---|---|
| BAS positive | 33 (7.3) |
| Carcinoma | 11 (2.5) |
| Atypical cells | 10 (2.2) |
| NSCLC | 6 (1.3) |
| Squamous | 5 (1.1) |
| Adenocarcinoma | 1 (0.2) |
| Diagnostic contribution | 1 (0.2) |
| BA microbiological | 30 (6.7) |
| Streptococcus pneumoniae | 6 (1.3) |
| Pseudomonas aeruginosa | 5 (1.1) |
| Haemophilus influenzae | 4 (0.9) |
| Enterobacteriaceae | 3 (0.7) |
| Klebsiella pneumoniae | 2 (0.4) |
| Moraxella catarrhalis | 2 (0.4) |
| Streptococcus salivarius | 2 (0.4) |
| Escherichia coli | 2 (0.4) |
| Citrobacter koseri | 1 (0.2) |
| Streptococcus parasanguinis | 1 (0.2) |
| Serratia marcescens | 1 (0.2) |
| Proteus mirabilis | 1 (0.2) |
| Tuberculosis Bacilloscopic positive | 2 (0.4) |
EBUS microbiological samples by transbronchial puncture were obtained in 52 (11.5%) with a suspected infection. Of them, a bacteria was detected in 8 (1.8%), 5 (1.1%) of them show mycobacterium tuberculosis. BA microbiological samples found germens in 30 (6.7%) patients. Of them, 16 (53.3%) have a previous bronchoscopy, and 11 (68.7%) have the same specimen in the previous bronchoscopy BA. Only in 5 cases (1.1%), BA of EBUS found microbiological specimens not detected in previous bronchoscopy. These 5 cases do not receive antibiotics and evolved correctly. Of the 30 patients with a bacteria detected on EBUS BA, 7 (23.3%) received antibiotic treatment and evolved correctly. The remaining 23 patients were asymptomatic or the specimens were considered to be upper airway contamination and did not receive antibiotics, and also evolved correctly.
Potential for Cost ReductionThe potential total cost reduction during the study period at our centre if the BA routine was eliminated in the 450 patients, is 12,375.00€ for cytological analysis, 9562.50€ for microbiological analysis, bringing the total cost of the two studies performed routinely to 21,937.50€.
DiscussionOur prospective study shows a low sensitivity of cytology specimens from EBUS-TBNA BA. Only in 1 (0.2%) case provided a previously unknown diagnosis (however, this patient did not have a previous bronchoscopy). To our knowledge, this is the first study that analyses the BA from EBUS.
Several studies in lung cancer patients analyse the diagnostic yield of bronchial washings (BW) in flexible bronchoscopy, with low diagnostic contributions (1.1%),8 and a diagnostic yield for bronchial washings of about 38%,9 even after forceps biopsy.10 The diagnostic yield increases in cases of endobronchial lesions in patients with lung cancers11 with an acceptable cost-effectiveness,12 even after transbronchial biopsy in guide sheath EBUS (EBUS-GS).13
Girard et al.,6 demonstrate a sensitivity of cytology specimens from bronchial aspirate or washing at about 15%, and have few cases of EBUS at the same time and after bronchial biopsies, and suggest avoiding the BA routinely. Our study was based only on EBUS and showed that the diagnostic yield drops to 0.1%. Even in that case, it was necessary to perform any other technique to obtain more samples for the molecular test. So, our results reinforce the idea of not performing BA routinely.
The development of new diagnostic tools such as EBUS should also decrease the “added value” of bronchial cytology.14,15 Also, the management of non-small cell lung cancer (NSCLC) now requires multiple molecular tests to guide the treatment strategy for an increasing number of targeted agents,16 and makes biopsies a priority over cytology,14,15,17 and cytological samples might not be appropriated.18
The EBUS-TBNA aspirate could be routinely sent for microbiological cultures, and many studies demonstrate the usefulness for the diagnosis of tuberculosis. BA may be useful to assess the microbiology of central airways secretions information when a dominant pathogen or resistant organism is identified,19,20 but it is necessary to use protected catheters to avoid contamination.7 Even so, there are no studies on the BA collected on EBUS. Our study shows a few cases in which the collection of microbiological BA was diagnostic (6.7%) but less than 25% of the patients received antibiotics and evolved correctly. In addition, more than half of the patients had a previous bronchoscopy and only in 31% of the cases, the microbiological isolation was different. Of them, none was treated with a favourable clinical evolution, which indicates the low relevance of microbiological BA.
In our study we have two cases of BA with mycobacterium tuberculosis, and the two cases had a tuberculosis suspicion, and mycobacterium tuberculosis in a PAAF. But in case of tuberculosis suspicion, depending on the geographical area of study, BA should be performed.
Although it is a prospective study with great sample size, it has certain limitations. The study was one centre study and this leads to a lack of external validation, especially in the microbiological results, which may vary depending on the geographical area of study. Moreover, there are no previous studies o consensus about BA in EBUS, so our study needs to be followed by new research.
Our study assesses the potential economic benefit of not performing routine BA in EBUS. Although the potential for cost savings in the care of an individual patient is modest, several centres perform routine BA annually, so potential cost savings could be significant. This results results should be adjusted for each region, based on costs.
ConclusionsIn conclusion, the low diagnostic yield and contribution of bronchial aspirate cytology specimens and microbiological cultures, supports the idea of not performing the routine BA during EBUS-TBNA. Larger studies could be necessary to confirm the findings and to be able to select the patients who benefit from the collection of this type of sample.
Conflict of InterestsThe authors state that they have no conflict of interests.