The aim of this study is to assess the diagnostic value of the magnetic resonance imaging (MRI) in differentiating metastasic from non-metastatic lymph nodes in NSCLC patients compared with computed tomography (CT) and fluorodeoxyglucose (FDG) - positron emission tomography (PET) or both combined.
MethodsTwenty-three studies (19 studies and 4 meta-analysis) with sample size ranging between 22 and 250 patients were included in this analysis. MRI, regardless of the sequence obtained, where used for the evaluation of N-staging of NSCLC. Histopathology results and clinical or imaging follow-up were used as the reference standard. Studies were excluded if the sample size was less than 20 cases, if less than 10 lymph nodes assessment were presented or studies where standard reference was not used. Papers not reporting sufficient data were also excluded.
ResultsAs compared to CT and PET, MRI demonstrated a higher sensitivity, specificity and diagnostic accuracy in the diagnosis of metastatic or non-metastatic lymph nodes in N-staging in NSCLC patients. No study considered MRI inferior than conventional techniques (CT, PET or PET/CT). Other outstanding results of this review are fewer false positives with MRI in comparison with PET, their superiority over PET/CT to detect non-resectable lung cancer, to diagnosing infiltration of adjacent structures or brain metastasis and detecting small nodules.
ConclusionMRI has shown at least similar or better results in diagnostic accuracy to differentiate metastatic from non-metastatic mediastinal lymph nodes. This suggests that MRI could play a significant role in mediastinal NSCLC staging.
El objetivo de este trabajo es evaluar el potencial diagnóstico de las imágenes por resonancia magnèc)tica (RM) para identificar nódulos linfáticos metastásicos frente aquellos no metastásicos en pacientes con cáncer de pulmón no microcítico (CPNM) en comparación con la tomografía computarizada (TC), la tomografía por emisión de positrones con 18F-fluorodesoxiglucosa (PET-FDG) o ambas tèc)cnicas combinadas.
Mèc)todosEn el análisis se incluyeron 23 estudios (19 estudios y 4 metaanálisis) con tamaños de muestra entre 22 y 250 pacientes. Para la estadificación ganglionar (N) del CPNM se utilizaron imágenes de RM independientemente de la secuencia obtenida. Como estándar de referencia se usaron los resultados histopatológicos y el seguimiento clínico o por imagen. Se excluyeron aquellos estudios con tamaños muestrales menores de 20 casos, aquellos con menos de 10 nódulos linfáticos evaluados o estudios en los que no se usó un estándar de referencia. Tambièc)n se excluyeron los artículos que no presentaron suficientes datos.
ResultadosSe observó que la RM presentaba mayor sensibilidad, especificidad y precisión en la estadificación ganglionar (N) y el diagnóstico de nódulos linfáticos metastásicos o no metastásicos en pacientes con CPNM en comparación con la TC y el PET. Ningún estudio consideró a la RM inferior con respecto a otras tèc)cnicas convencionales (TC, PET y PET/TC). Otros resultados destacables de esta revisión son que con la RM se originaron menos falsos positivos en comparación con el PET, y su superioridad respecto al PET/TC en la detección de tumores de pulmón no operables, en el diagnóstico de infiltración en estructuras adyacentes o metástasis cerebrales, así como en la detección de nódulos de pequeño tamaño.
ConclusiónLa RM dio lugar a mejores resultados o, al menos comparables, relacionados con la precisión diagnóstica para diferenciar nódulos linfáticos metastásicos de no metastásicos. Esto sugiere que la RM podría jugar un papel importante en la estadificación mediastinal en pacientes con CPNM.
Lung cancer is a major health problem worldwide and the most common cause of cancer death in developed countries.1 In 2017 lung cancer is expected to be the second most frequent tumor in males and women and it will continue being the most frequent cause of cancer death.2 Tobacco use is the largest preventable cause of cancer.3 Up to 10-15% of all lung cancers occur in never smokers 4 and radon is the second risk factor of lung cancer in never-smokers.5
The TNM classification is the cancer staging system used to describe the anatomical scope of a tumor according to three components. Recently, the International Association for the Study of Lung Cancer (IASLC) has published the 8th edition.6 Nodal (N) status is important for prognosis and an adequate staging is essential for a subsequent optimal therapeutic approach.
Noninvasive techniques for mediastinal lymph node staging include computed tomography (CT), combined or not with fluorodeoxyglucose (FDG) - positron emission tomography (PET), PET/CT. Since 1980s, the possible role of magnetic resonance imaging (MRI) has been investigated.7 Traditionally, CT has been the main technique for N staging, but it is limited by a low sensitivity (55-65%) and specificity (65-75%) because it only uses size criteria (an axial short-axis diameter of 1cm or greater) or abnormal shape or attenuation of the lymph node to suspect metastasic involvement.8
PET has been validated as a technique which is superior to CT when differentiating metastatic or non-metastatic lymph nodes as it provides metabolic information based on the glucose consumption from tumor cells. Its sensitivity and specificity are higher than CT but are still greater when both techniques are combined (PET/CT).9 However, PET has associated diagnostic limitations by confounding malignancy from inflammatory changes resulting in false positives and is limited by spatial resolution,9 as well as difficulties in detecting certain tumors whose lesions may be PET-negative (adenocarcinoma, carcinoid…), resulting in false negatives.10
Recent studies have reported that magnetic resonance imaging (MRI) could be useful for N-staging in non-small cell lung cancer (NSCLC) compared to conventional techniques (CT, PET, PET/CT)11 by differentiating metastasic from non-metastasic nodes. Those studies have also shown that certain whole-body MRI sequences as short time inversion recovery (STIR) turbo spin-echo (SE) or diffusion weighted (DW) MRI are superior than conventional sequences (T1-weighted MR, T2-weighted MR) or echo planar imaging sequence (EPI-DWI).12 Once MRI images are obtained, a detailed study of them can be made from the qualitative or quantitative point of view. To determine the qualitative analysis, two radiologists are required to interpret the MR images and evaluate the probability that a lymph node contains metastasis using a five-point visual scoring system. Qualitative analysis depends on the consensus of the radiologists who have analyzed the Images.12,13 Using the quantitative analysis all signal intensity (SI) of lymph nodes are normalized by comparing them with the signal intensity of the 0,9% saline panthom to produce the lymph node saline ratio (LSR).13 The rationale for detecting a positive nodule is that malignant tumors have more cellularity and less extracellular space than normal tissue, resulting in higher SI values. DW MRI bases the analysis of the images trough water molecules movement in biological tissues (Brownian movement), taking into account that in malignant tumors and those regions affected by metastasis will have restricted water molecules movement than normal, resulting in a decreased apparent diffusion coefficient (ADC).14
The purpose of the present study is to perform a systematic review to assess the overall diagnostic value of MRI to discriminate between metastatic and non-metastatic lymph nodes in primary NSCLC patients in comparison with PET/CT.
Material and methodsLiterature researchA literature research was performed in PubMed (Medline), EMBASE and Cochrane databases. To retrieve information the following search strategy was employed in PubMed using a combination of MeSH terms (“Magnetic Resonance Imaging” [Mesh] AND “Lung Neoplasms” [Mesh]). AND “Mediastinal staging” [Mesh] AND “Clinical staging” [Mesh] AND “Non-invasive” [Mesh] AND “NSCLC” [Mesh]. The following limits were used: language (English or Spanish), humans and publications dating from 01/01/2007 to 15/08/2017. The reference lists of identified articles were also manually searched to obtain additional papers. The reports found to be eligible on the basis of their title and, subsequently, from the abstract, were then selected to further determine suitability for inclusion in the present study.
Inclusion and exclusion criteriaEligible studies were reviewed and included in this systematic review according to the following inclusion criteria: MRI was used for the evaluation of N staging of lung cancer. Sufficient information regarding true-positive (TP), false-positive (FP), true-negative (TN) and false-negative (FN) values could be identified or calculated from data in the original articles. Studies were excluded if the sample size was less than 20 cases, if less than 10 lymph nodes assessment were presented or studies where standard reference was not used. The only accepted standard reference was histology, excluding those articles that were used as standard reference clinical or imaging follow-up. Histopathology results were determined by percutaneous fine needle aspiration (PCNA), endobronchial ultrasound-guided transbronchial needle aspiration (EBUS), mediastinoscopy or after surgical lymph nodes resection through thoracotomy.
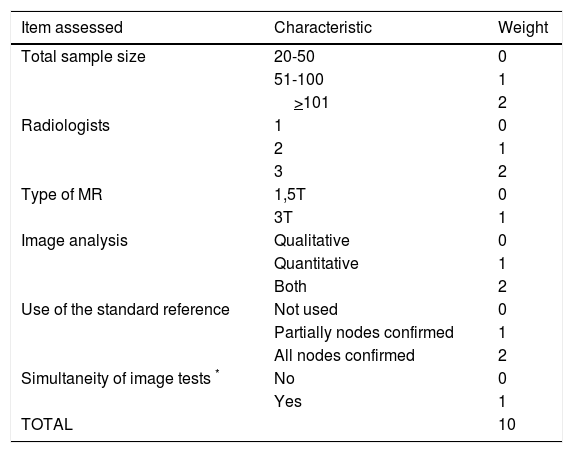
Data extraction and Quality AssessmentSearch results were checked by one reviewer and if there were doubts regarding the inclusion or exclusion of a certain paper, this was solved by consensus by all authors. We developed a quality scale to score the quality of the included studies. Similar scales have been used previously by other groups when performing other systematic reviews.15,16 We considered 6 items to assess the quality of included studies which are shown in Table 1. Each item opted a score between 0 and 1 to 3 points (the higher the score the higher the quality).
Quality score to assess the included studies.
| Item assessed | Characteristic | Weight |
|---|---|---|
| Total sample size | 20-50 | 0 |
| 51-100 | 1 | |
| >101 | 2 | |
| Radiologists | 1 | 0 |
| 2 | 1 | |
| 3 | 2 | |
| Type of MR | 1,5T | 0 |
| 3T | 1 | |
| Image analysis | Qualitative | 0 |
| Quantitative | 1 | |
| Both | 2 | |
| Use of the standard reference | Not used | 0 |
| Partially nodes confirmed | 1 | |
| All nodes confirmed | 2 | |
| Simultaneity of image tests * | No | 0 |
| Yes | 1 | |
| TOTAL | 10 |
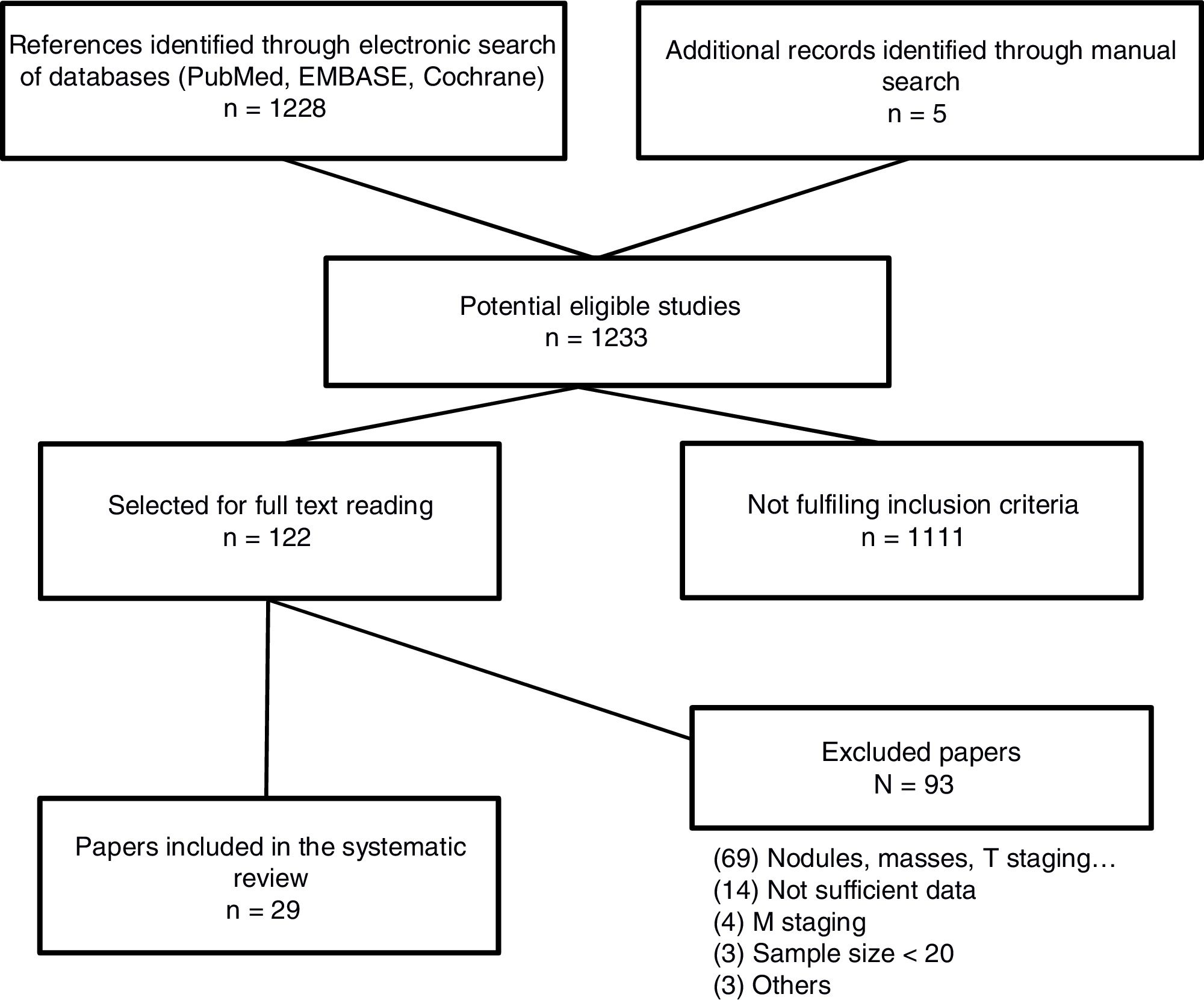
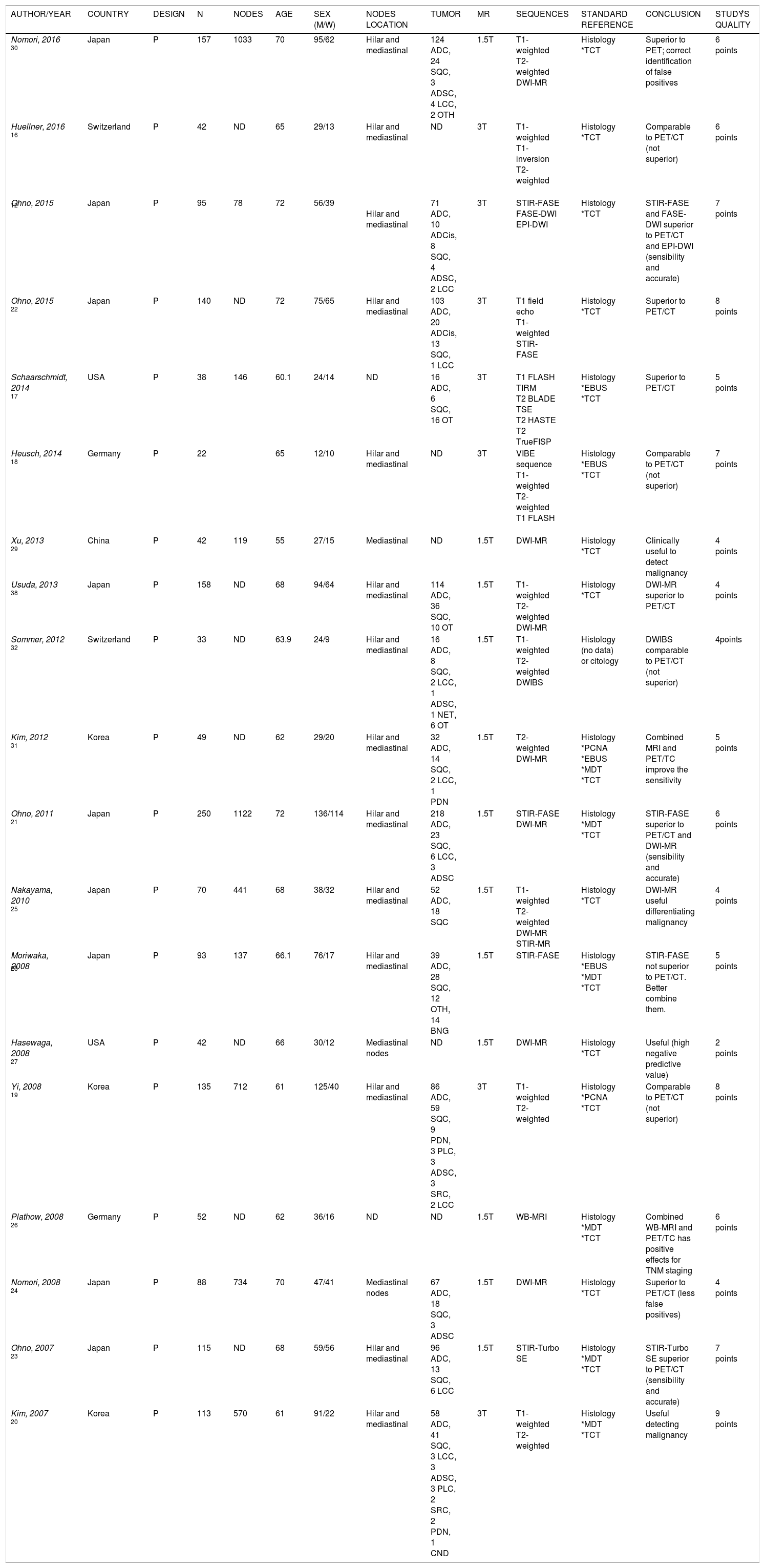
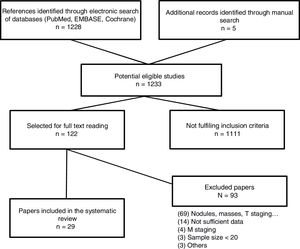
The searh resulted in 1,234 studies. Of these 123, were selected for full text reading, and 23 (19 prospective studies and 4 meta-analysis) were finally included. The most frequent exclusion criterion was the study of lung lesions (solitary pulmonary nodule and masses) that did not include lymph nodes. The flowchart of the search is shown in Figure 1. Sample size is ranged between 22 and 250 patients. After careful reading of the studies, we could observe that over the last 10 years different MRI sequences for the study of lymph nodes have been used. We have focused the results in those sequences which have provided the most relevant results (STIR turbo SE and DWI MRI). Only 5 studies used other MRI sequences.16•20
Prospective and retrospective studies tm) resultsThe largest prospective study published on N-stage assessment in patients with NSCLC was published by OHNO et al.21 in 2011 including 250 patients with mediastinal and hilar lymph nodes studied by STIR FASE imaging, DW MR imaging and FDG PET/CT. In both quantitative and qualitative N-stage assessment, STIR turbo SE showed more sensibility and accuracy (82.8% and 86.8%, respectively, in quantitative assessment; 77.4% and 84.4%, respectively, in qualitative assessment) compared to DW MR imaging or FDG PET/CT. The same group carried out the study with the highest quality,22 which included 140 consecutive patients. The capabilities for TNM classification and the assessment of clinical stage and tumor resectability among whole-body MRI were compared, coregistering PET/MR imaging with or without SI assessment, and FDG PET/CT. It was shown that the capability to assess tumor resectability and accuracy of whole-body MRI and PET/MRI with SI assessment (97.1%) was significantly higher than that of PET/MRI without SI assessment and FDG PET/CT (85%; p<0.001). In other studies by OHNO et al.12,23 it has been observed that sensitivity and diagnostic accuracy was higher with MRI (STIR turbo SE and fast advantage spin-echo sequence (FASE-DWI)12) compared with FDG PET/CT both for quantitative23 and qualitative12 analysis.
Other published studies24•30 comparing MRI sequences (fundamentally DWI-MRI and STIR turbo SE sequences) and FDG PET/CT, showed similar results. Both sensitivity and diagnostic accuracy25 were higher with MRI sequences than with PET/CT, in addition a high negative predictive value (NPV) of DW-MRI (97%).27 These studies postulated MRI as a technique that could correctly differentiate metastasic from non-metastasic nodes and showed fewer false-positives than PET in the mediastinum (p=0.011) and in normal or enlarged lymph nodes. No statistically significant differences were observed between DWI-MRI and STIR turbo SE.25
There were three studies26,28,31 comparing MRI with FDG PET/CT for preoperative -nodal staging in NSCLC (with 93, 52 and 49 patients, respectively). Diagnostic accuracy of MRI was not significantly higher than that of FDG PET/CT, although they concluded that it had fewer false positives, and probably the best option would be to combine both methods for an adequate preoperative staging, even avoiding the use of invasive diagnostic techniques.
One study32 with 33 patients compared prospectively the diagnostic efficacy of MRI with FDG PET/CT. Results showed that MRI was a feasible technique for the assessment of NSCLC, comparable but not superior to FDG PET/CT in N-staging.
Characteristics of included studies are summarized in Table 2 and Table 3.
Characteristics of included studies (19 prospective studies; does not include meta-analysis).
| AUTHOR/YEAR | COUNTRY | DESIGN | N | NODES | AGE | SEX (M/W) | NODES LOCATION | TUMOR | MR | SEQUENCES | STANDARD REFERENCE | CONCLUSION | STUDÝS QUALITY |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nomori, 2016 30 | Japan | P | 157 | 1033 | 70 | 95/62 | Hilar and mediastinal | 124 ADC, 24 SQC, 3 ADSC, 4 LCC, 2 OTH | 1.5T | T1-weighted T2-weighted DWI-MR | Histology *TCT | Superior to PET; correct identification of false positives | 6 points |
| Huellner, 2016 16 | Switzerland | P | 42 | ND | 65 | 29/13 | Hilar and mediastinal | ND | 3T | T1-weighted T1-inversion T2-weighted | Histology *TCT | Comparable to PET/CT (not superior) | 6 points |
| Ohno, 2015 12 | Japan | P | 95 | 78 | 72 | 56/39 | Hilar and mediastinal | 71 ADC, 10 ADCis, 8 SQC, 4 ADSC, 2 LCC | 3T | STIR-FASE FASE-DWI EPI-DWI | Histology *TCT | STIR-FASE and FASE-DWI superior to PET/CT and EPI-DWI (sensibility and accurate) | 7 points |
| Ohno, 2015 22 | Japan | P | 140 | ND | 72 | 75/65 | Hilar and mediastinal | 103 ADC, 20 ADCis, 13 SQC, 1 LCC | 3T | T1 field echo T1-weighted STIR-FASE | Histology *TCT | Superior to PET/CT | 8 points |
| Schaarschmidt, 2014 17 | USA | P | 38 | 146 | 60.1 | 24/14 | ND | 16 ADC, 6 SQC, 16 OT | 3T | T1 FLASH TIRM T2 BLADE TSE T2 HASTE T2 TrueFISP | Histology *EBUS *TCT | Superior to PET/CT | 5 points |
| Heusch, 2014 18 | Germany | P | 22 | 65 | 12/10 | Hilar and mediastinal | ND | 3T | VIBE sequence T1-weighted T2-weighted T1 FLASH | Histology *EBUS *TCT | Comparable to PET/CT (not superior) | 7 points | |
| Xu, 2013 29 | China | P | 42 | 119 | 55 | 27/15 | Mediastinal | ND | 1.5T | DWI-MR | Histology *TCT | Clinically useful to detect malignancy | 4 points |
| Usuda, 2013 38 | Japan | P | 158 | ND | 68 | 94/64 | Hilar and mediastinal | 114 ADC, 36 SQC, 10 OT | 1.5T | T1-weighted T2-weighted DWI-MR | Histology *TCT | DWI-MR superior to PET/CT | 4 points |
| Sommer, 2012 32 | Switzerland | P | 33 | ND | 63.9 | 24/9 | Hilar and mediastinal | 16 ADC, 8 SQC, 2 LCC, 1 ADSC, 1 NET, 6 OT | 1.5T | T1-weighted T2-weighted DWIBS | Histology (no data) or citology | DWIBS comparable to PET/CT (not superior) | 4points |
| Kim, 2012 31 | Korea | P | 49 | ND | 62 | 29/20 | Hilar and mediastinal | 32 ADC, 14 SQC, 2 LCC, 1 PDN | 1.5T | T2-weighted DWI-MR | Histology *PCNA *EBUS *MDT *TCT | Combined MRI and PET/TC improve the sensitivity | 5 points |
| Ohno, 2011 21 | Japan | P | 250 | 1122 | 72 | 136/114 | Hilar and mediastinal | 218 ADC, 23 SQC, 6 LCC, 3 ADSC | 1.5T | STIR-FASE DWI-MR | Histology *MDT *TCT | STIR-FASE superior to PET/CT and DWI-MR (sensibility and accurate) | 6 points |
| Nakayama, 2010 25 | Japan | P | 70 | 441 | 68 | 38/32 | Hilar and mediastinal | 52 ADC, 18 SQC | 1.5T | T1-weighted T2-weighted DWI-MR STIR-MR | Histology *TCT | DWI-MR useful differentiating malignancy | 4 points |
| Moriwaka, 2008 28 | Japan | P | 93 | 137 | 66.1 | 76/17 | Hilar and mediastinal | 39 ADC, 28 SQC, 12 OTH, 14 BNG | 1.5T | STIR-FASE | Histology *EBUS *MDT *TCT | STIR-FASE not superior to PET/CT. Better combine them. | 5 points |
| Hasewaga, 2008 27 | USA | P | 42 | ND | 66 | 30/12 | Mediastinal nodes | ND | 1.5T | DWI-MR | Histology *TCT | Useful (high negative predictive value) | 2 points |
| Yi, 2008 19 | Korea | P | 135 | 712 | 61 | 125/40 | Hilar and mediastinal | 86 ADC, 59 SQC, 9 PDN, 3 PLC, 3 ADSC, 3 SRC, 2 LCC | 3T | T1-weighted T2-weighted | Histology *PCNA *TCT | Comparable to PET/CT (not superior) | 8 points |
| Plathow, 2008 26 | Germany | P | 52 | ND | 62 | 36/16 | ND | ND | 1.5T | WB-MRI | Histology *MDT *TCT | Combined WB-MRI and PET/TC has positive effects for TNM staging | 6 points |
| Nomori, 2008 24 | Japan | P | 88 | 734 | 70 | 47/41 | Mediastinal nodes | 67 ADC, 18 SQC, 3 ADSC | 1.5T | DWI-MR | Histology *TCT | Superior to PET/CT (less false positives) | 4 points |
| Ohno, 2007 23 | Japan | P | 115 | ND | 68 | 59/56 | Hilar and mediastinal | 96 ADC, 13 SQC, 6 LCC | 1.5T | STIR-Turbo SE | Histology *MDT *TCT | STIR-Turbo SE superior to PET/CT (sensibility and accurate) | 7 points |
| Kim, 2007 20 | Korea | P | 113 | 570 | 61 | 91/22 | Hilar and mediastinal | 58 ADC, 41 SQC, 3 LCC, 3 ADSC, 3 PLC, 2 SRC, 2 PDN, 1 CND | 3T | T1-weighted T2-weighted | Histology *MDT *TCT | Useful detecting malignancy | 9 points |
ADC: adenocarcinoma. ADCis: adenocarcinoma in situ. ADSC: adenosquamous. BLADE: proprietary name for periodically rotated overlapping parallel lines with enhanced reconstruction [PROPELLER] in MR systems. BNG: benign. CND: carcinoid. DWI-MR: diffusion weighted imaging magnetic resonance. DWIBS: single shot echo planar imaging. EBUS: endobronchial ultrasound-guided transbronchial needle aspiration. FLASH: fast low angle shot gradient echo sequence. HASTE: half fourier acquired single short turbo spin echo sequence. LCC: large cell carcinoma. M: male. MDT: mediastinoscopy. MEC: mucoepidermoid carcinoma. MR: magnetic resonance. MX: mixed small cell carcinoma and adenocarcinoma. NET: neuroendocrine tumor. NSCLC: non-small cell lung cancer. OTH: others. P: prospective. PCNA: percutaneous fine needle aspiration. PDN: poorly differentiated non-small cell lung cancer. PLC: pleomorphic carcinoma. R: retrospective. SCC: small cell carcinoma. SQC: squamous cell carcinoma. SRC: sarcomatoid carcinoma. TCT: thoracotomy.
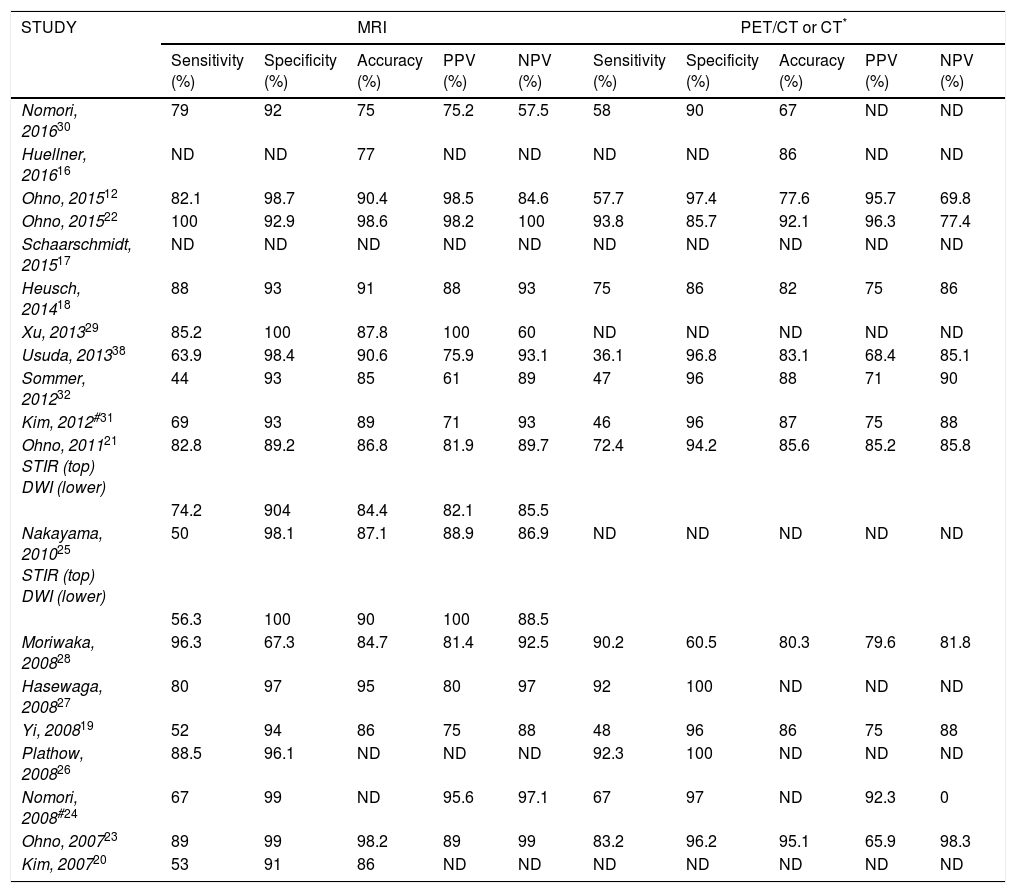
Comparison of diagnostic performance for lymph node metastasis by all methods.
| STUDY | MRI | PET/CT or CT* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | Accuracy (%) | PPV (%) | NPV (%) | Sensitivity (%) | Specificity (%) | Accuracy (%) | PPV (%) | NPV (%) | |
| Nomori, 201630 | 79 | 92 | 75 | 75.2 | 57.5 | 58 | 90 | 67 | ND | ND |
| Huellner, 201616 | ND | ND | 77 | ND | ND | ND | ND | 86 | ND | ND |
| Ohno, 201512 | 82.1 | 98.7 | 90.4 | 98.5 | 84.6 | 57.7 | 97.4 | 77.6 | 95.7 | 69.8 |
| Ohno, 201522 | 100 | 92.9 | 98.6 | 98.2 | 100 | 93.8 | 85.7 | 92.1 | 96.3 | 77.4 |
| Schaarschmidt, 201517 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Heusch, 201418 | 88 | 93 | 91 | 88 | 93 | 75 | 86 | 82 | 75 | 86 |
| Xu, 201329 | 85.2 | 100 | 87.8 | 100 | 60 | ND | ND | ND | ND | ND |
| Usuda, 201338 | 63.9 | 98.4 | 90.6 | 75.9 | 93.1 | 36.1 | 96.8 | 83.1 | 68.4 | 85.1 |
| Sommer, 201232 | 44 | 93 | 85 | 61 | 89 | 47 | 96 | 88 | 71 | 90 |
| Kim, 2012#31 | 69 | 93 | 89 | 71 | 93 | 46 | 96 | 87 | 75 | 88 |
| Ohno, 201121 STIR (top) DWI (lower) | 82.8 | 89.2 | 86.8 | 81.9 | 89.7 | 72.4 | 94.2 | 85.6 | 85.2 | 85.8 |
| 74.2 | 904 | 84.4 | 82.1 | 85.5 | ||||||
| Nakayama, 201025 STIR (top) DWI (lower) | 50 | 98.1 | 87.1 | 88.9 | 86.9 | ND | ND | ND | ND | ND |
| 56.3 | 100 | 90 | 100 | 88.5 | ||||||
| Moriwaka, 200828 | 96.3 | 67.3 | 84.7 | 81.4 | 92.5 | 90.2 | 60.5 | 80.3 | 79.6 | 81.8 |
| Hasewaga, 200827 | 80 | 97 | 95 | 80 | 97 | 92 | 100 | ND | ND | ND |
| Yi, 200819 | 52 | 94 | 86 | 75 | 88 | 48 | 96 | 86 | 75 | 88 |
| Plathow, 200826 | 88.5 | 96.1 | ND | ND | ND | 92.3 | 100 | ND | ND | ND |
| Nomori, 2008#24 | 67 | 99 | ND | 95.6 | 97.1 | 67 | 97 | ND | 92.3 | 0 |
| Ohno, 200723 | 89 | 99 | 98.2 | 89 | 99 | 83.2 | 96.2 | 95.1 | 65.9 | 98.3 |
| Kim, 200720 | 53 | 91 | 86 | ND | ND | ND | ND | ND | ND | ND |
Four meta-analysis with 12, 10, 18 and 19 studies included, respectively, had been published11,33•35 comparing the diagnostic value of MRI in N staging of NSCLC which agreed on its high specificity and diagnostic accuracy in detecting metastasic lymph nodes in patients with lung cancer. These studies had restrictive inclusion criteria and were focused in a specific type of MRI. No study reported serious complications.
Study qualityThe scoring of the included studies ranged between 0 and 10 points. The highest quality was for three studies with more than 100 patients. The mean scoring of the included studies was 5.6 points. Table 2 presents in detail the quality of each study.
DiscussionThe results of this systematic review point out that MRI, especially in some sequences such as STIR turbo SE and DW-MRI, has a high diagnostic accuracy to diagnose metastasic or non-metastasic lymph nodes in N-staging in non-small cell lung cancer patients. These studies specially highlighted its high sensitivity, specifity and diagnostic accuracy. In comparison with conventional techniques such as combined FDG PET/CT or both techniques separately, some studies considered MRI sequences superior for N-staging, and some other studies considered MRI sequences comparable but not superior to conventional techniques. No study considered MRI inferior than conventional techniques. Even in those studies where MRI was comparable but not superior to FDG PET/CT, MRI had fewer false positives.
Knowing that MRI has a useful role in the preoperative mediastinal and hiliar nodal staging, the next step is where this imaging test may fit in clinical practice. Some authors speculate that MRI sequences are superior to FDG PET/CT and can be used in place of it as an alternative in the clinical practice.17,27 Other study 32,34,24 support that MRI and FDG PET/CT, would provide complementary information, so using them together without eliminating or replacing any test as part of the usual protocol would improve significantly diagnostic capability for N-staging28 and making it less necessary to use invasive diagnostic techniques.31 Finally, the remaining studies, regardless of whether they conclude that MRI is superior or similar than FDG PET/CT, do not make recommendations on the ideal time to perform the MRI in the preoperative staging, whether it should replace some conventional test, or if otherwise, it would be better to combine them.
Some advantages of MRI should be highlighted: 1) there is no radiation exposure; 2) patients do not have to fast before examination; 3) less test time (30minutes for DWI or STIR vs. 90minutes for PET/CT); 4) less test cost;24 5) administration of contrast medium is not necessary;27 6) easier accessibility to MRI because not all hospitals have PET/CT.14 MRI is a simple technique available in most hospitals that allows performing between 5-20 sequences, each of which provides a type of information about tissues (anatomical or functional). In some of them, as in case of STIR sequence that is performed with cardiac synchronization, the use of some software that usually comes integrated in the resonance device itself may be required. It should also be noted that there are also disadvantages: presence of motion artifacts (breathing, cardiac movement, pulsations...) or other artifacts as metallic bodies, tatoos, prostheses, surgical clips, etc. They might negatively affect the quality of lung image. Claustrophobia is the main inconvenient of this technique.36
Methodological shortcomings of the available literatureDifferent methodological problems continue to hinder MRI use in clinical practice. Radiologists and nuclear medicine physicians have more experience and availability with FDG PET/CT images than MRI37 probably because their use has not been sufficiently encouraged or protocols have not been developed. It would be convenient to investigate which sequence of MRIs are more useful for mediastinal staging with multicenter comparative studies and establish an optimal acquisition protocol that has not been defined currently. It is also necessary to develop a standard acquisition protocol as a routine clinical application.33 The methods that have been used until now to differentiate metastatic from non-metastatic lesions have been the quantitative (ADC value) and qualitative (visual score) methods. Although no significant differences have been demonstrated between them, the sensitivity and accuracy of quantitative methods has been found to be slightly higher compared to the qualitative method (93% and 87% vs. 88% and 86%, respectively).13 Tumor lesions are known to have a decreased ADC value; however, cut off values have not been established to clearly differentiate between metastatic and non-metastatic lymph nodes to date.13 It is important to keep in mind that specifically lesions (necrotic lesions, mucinous carcinoma or small nodules) could not be diagnosed by MRI. For example, ADC value for necrotic lesions could be lower than lesions without necrosis. Mucinous carcinoma, with its hypointense nature, could incurr in false negative because it behaves with high ADC values.38 Nodules smaller than 3mm cannot be detected by MRI38 and between 4-6mm23,25,29 could go unnoticed even if they have micrometastatic foci.
Other published reviewsThe published meta-analyses did not offer a global view of MRI and this was the reason for performing the present review. SHEN et al.34 included in their meta-analysis 18 studies but they only studied DW-MRI sequence. CHEN et al.33 only studied DW-MRI sequence too. The conventional method of usual clinical practice with which MRI was compared was PET or CT separately and, in few studies with PET/CT (both combined). WU et al.35 included 19 studies where they compared DW-MRI with FDG PET/CT but only in 3 of the 19 studies patients underwent DW-MRI, in the remaining 16, they only underwent FDG PET/CT. Finally, PEERLINGS et al.11 included only 12 studies with very strict selection criteria. For this reason, we considered to carry out this systematic review to offer a larger number of studies, with a broader view and considering all MRI sequences for the mediastinal lymph nodes study.
Recently, the first screening of lung cancer using low-dose CT (LDCT) compared to MRI has been published, and suitable results have been obtained in favor of MRI with an excellent sensitivity and specificity for the detection of nodules compared to LDCT. These results seem to reaffirm the promising use of resonance in the study of this type of neoplasms.39
ConclusionWith the studies available, it can be concluded than MRI, and specially DWI sequence, seems to play a relevant role in the N-staging of patients with NSCLC, compared to conventional methods (CT, PET, separately and both combined). MRI has shown at least similar or better results in diagnostic accuracy to differentiate metastatic from non-metastatic lymph nodes. Conversely, it cannot be established with the current evidence which would be the best time to do it, if it could replace any of the usual imaging tests or if it could be combined with them.
It would be convenient to validate a resonance protocol, as well as the standardization of ADC values to differentiate between malignant and benign lesions. In the near future, additional prospective and multicenter studies are warranted to confirm the clinical role of MRI in the staging of lung cancer.
Authors contributionTara Pereiro. Conception of the work. Data collection. Data analysis and interpretation. Drafting the article. Critical revision of the article. Final approval of the version to be published.
Alberto Ruano. Conception of the work. Drafting the article. Critical revision of the article. Final approval of the version to be published.
Josèc) Martín Carreira. Critical revision of the article. Final approval of the version to be published.
Antonio Golpe. Critical revision of the article. Final approval of the version to be published.
Anxo Martínez. Critical revision of the article. Final approval of the version to be published.
Luis Valdèc)s. Conception of the work. Drafting the article. Critical revision of the article. Final approval of the version to be published.
Conflicto de interesesLos autores declaran no tener ningún conflicto de intereses.