Although obstructive sleep apnea (OSA) is a prevalent condition among patients with acute coronary syndrome (ACS), the impact of OSA on cardiovascular event (CVE) recurrence is not homogeneous. We previously defined a specific phenotype of first-ACS patients without previous cardiovascular disease who are at increased risk of OSA-related CVE recurrence. However, the pathobiological mechanisms whereby OSA leads to adverse cardiovascular outcomes in this singular ACS phenotype remain to be investigated.
ObjectiveTo characterize the molecular pathways that relate OSA with CVE recurrence.
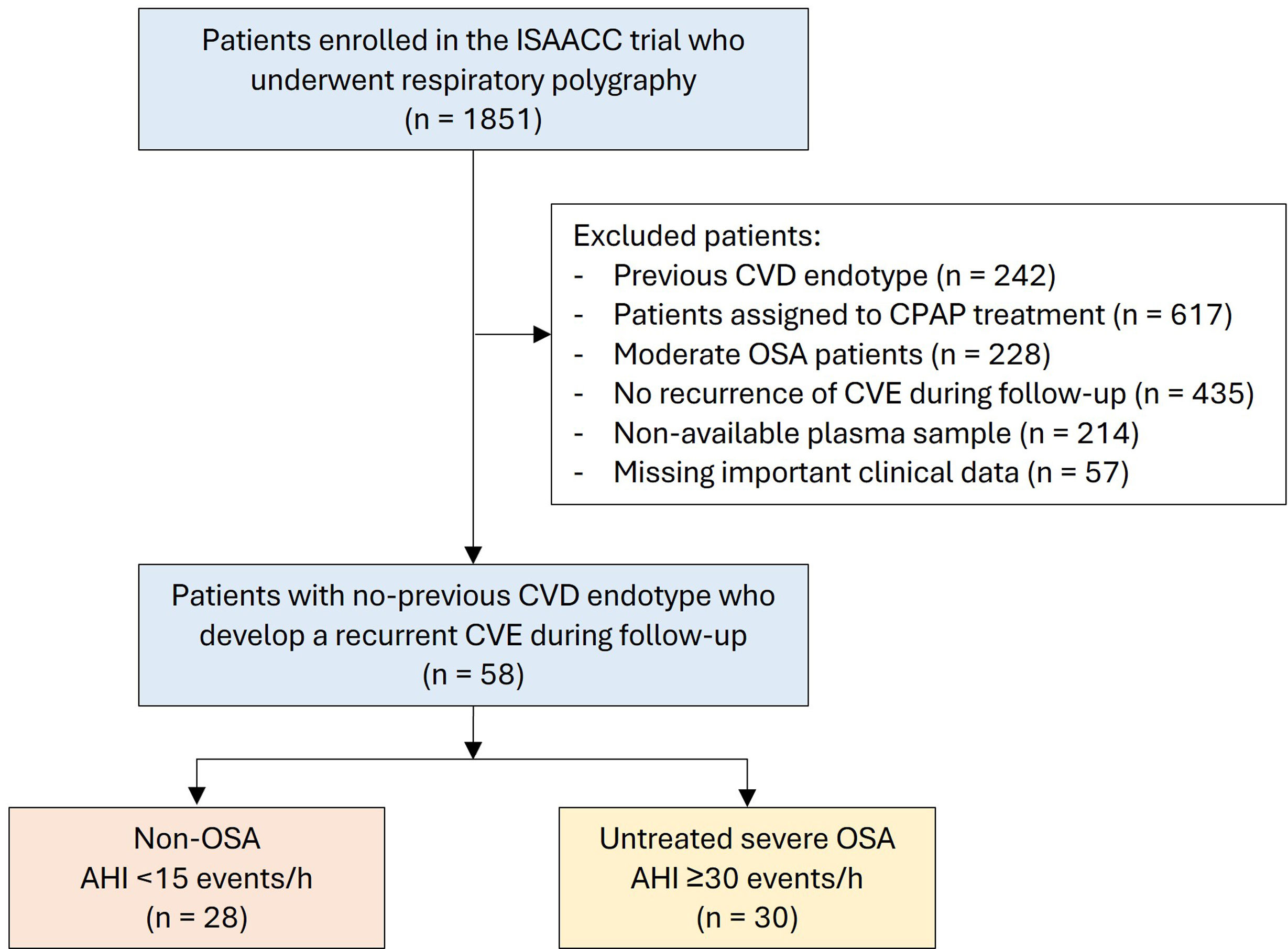
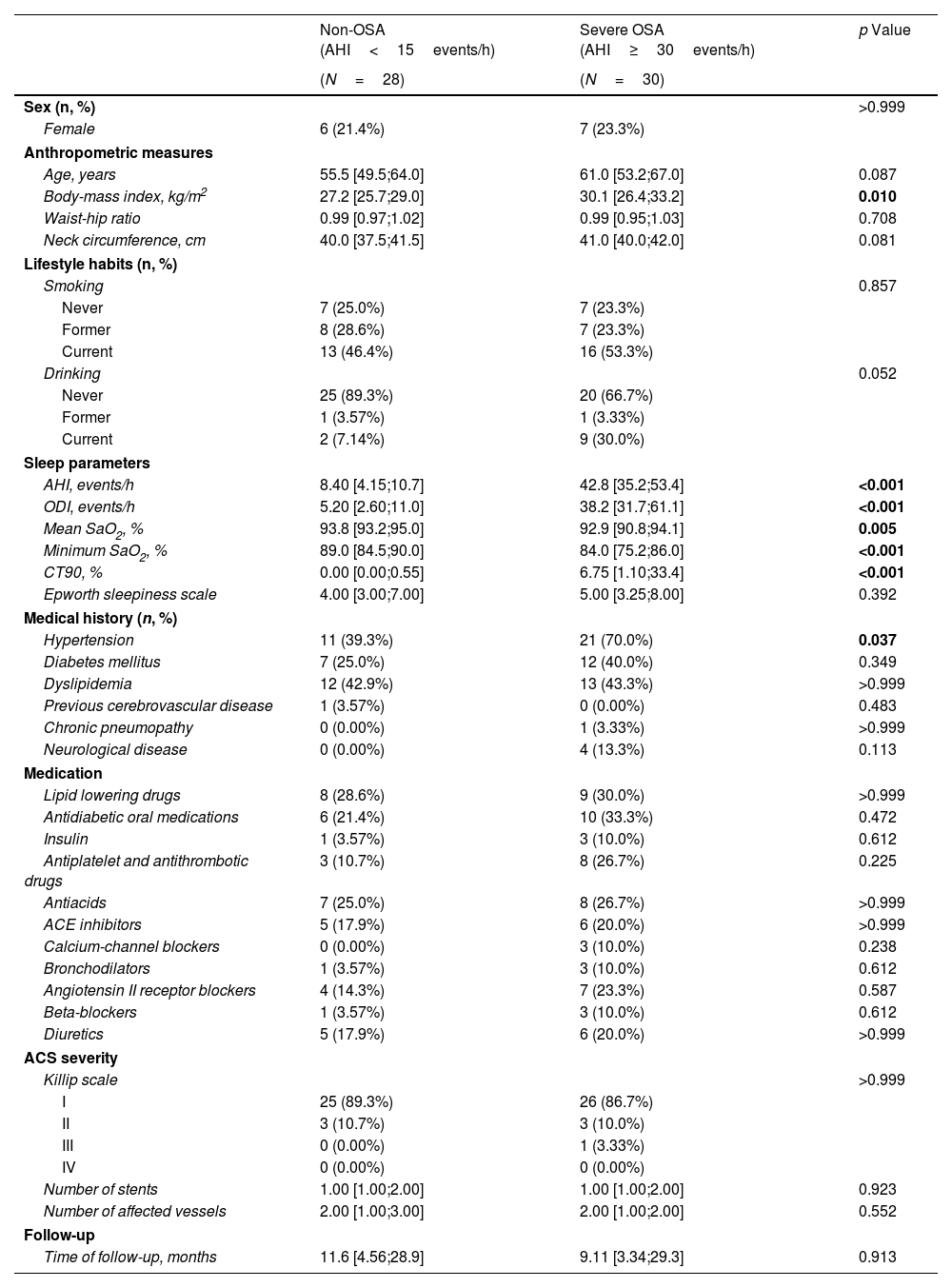
MethodsThis post hoc analysis of the ISAACC study (NCT01335087) included subjects without previous cardiovascular disease who were hospitalized for a first ACS and developed a recurrent CVE during the follow-up. Patients underwent respiratory polygraphy and fasting blood extraction during hospitalization. Two study groups were established on the basis of the apnea–hypopnea index (AHI): untreated severe OSA (AHI≥30events/h) and non-OSA (AHI<15events/h) groups. Proteomic profiling analysis included 276 cardiovascular and inflammatory-related plasma proteins via Olink® technology.
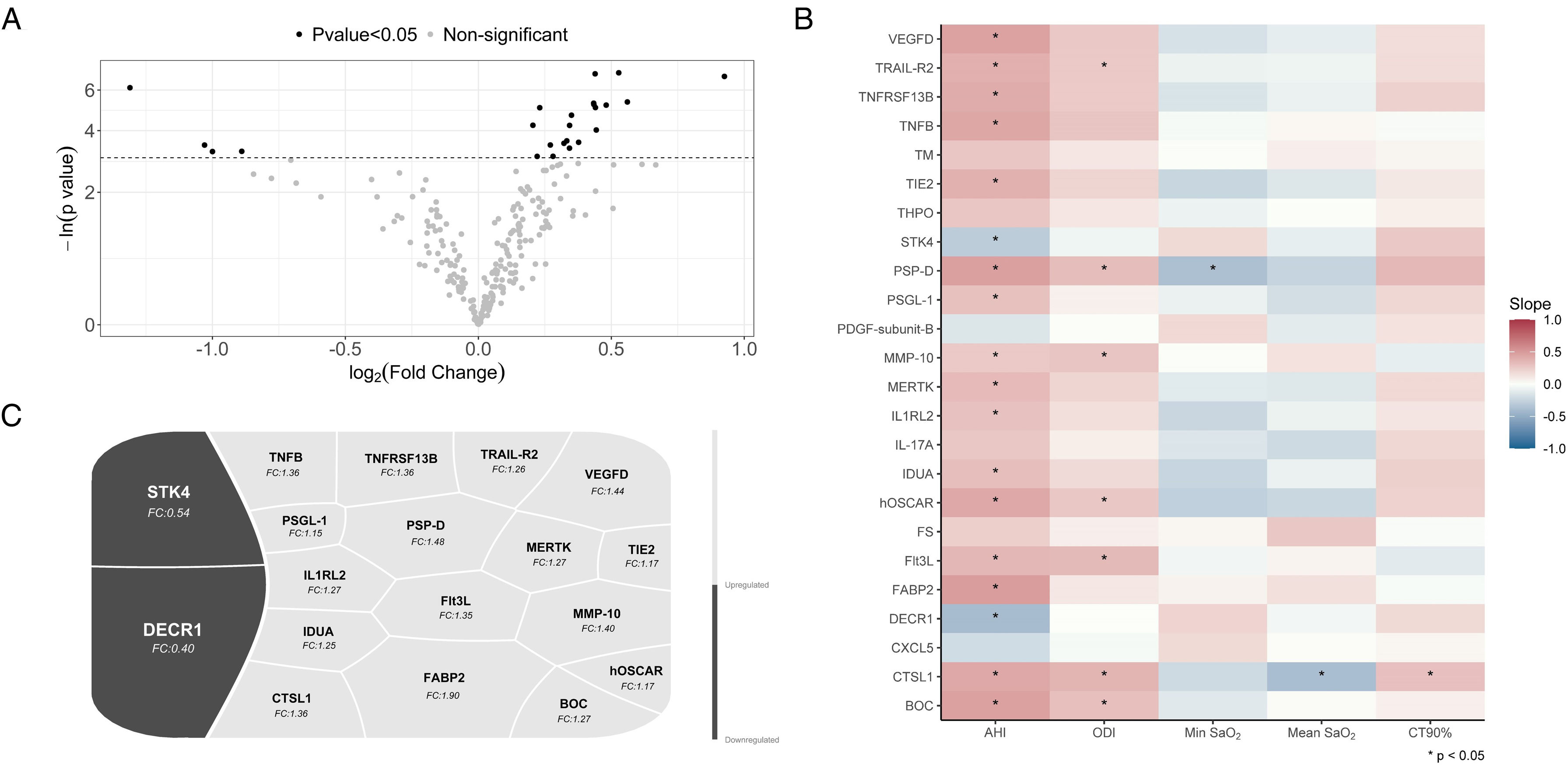
ResultsProteomics was performed in 58 patients (77.6% male, median [p25;p75] age 58.0 [51.2;65.8] years, and median BMI 28.6 [25.8;31.2]kg/m2). Thirty patients had severe OSA, and 28 subjects were considered non-OSA controls. A total of 24 plasma proteins were differentially expressed between the groups. Among these proteins, 18 were significantly associated with OSA severity parameters derived from respiratory polygraphy. Further bioinformatic analyses of OSA-related proteins revealed their involvement in several molecular pathways, mostly related to immune function, cell signaling, and inflammatory processes.
ConclusionA specific proteomic profile related to OSA presence and severity was identified in the plasma of ACS patients who developed recurrent CVEs. This analysis suggests the activation of key OSA-mediated molecular pathways with potential implications for cardiovascular prognosis.