Treatment with LABA/LAMA is recommended in GOLD B patients. We hypothesized that triple therapy (LABA/LAMA/ICS) will be superior to LABA/LAMA in achieving and maintaining clinical control (CC), a composite outcome that considers both impact and disease stability in a subgroup of GOLD B patients (here termed GOLD B+ patients) characterized by: (1) remaining symptomatic (CAT≥10) despite regular LABA/LAMA therapy; (2) having suffered one moderate exacerbation in the previous year; and (3) having blood eosinophil counts (BEC) ≥150cells/μL.
MethodsThe ANTES B+ study is a prospective, multicenter, open label, randomized, pragmatic, controlled trial designed to test this hypothesis. It will randomize 1028 B+ patients to continue with their usual LABA/LAMA combination prescribed by their attending physician or to begin fluticasone furoate (FF) 92μg/umeclidinium (UMEC) 55μg/vilanterol (VI) 22μg in a single inhaler q.d. for 12 months. The primary efficacy outcome will be the level of CC achieved. Secondary outcomes include the clinical important deterioration index (CID), annual rate of exacerbations, and FEV1. Exploratory objectives include the interaction of BEC and smoking status, all-cause mortality and proportion of patients on LABA/LAMA arm that switch therapy arms. Safety analysis include adverse events and incidence of pneumonia.
ResultsThe first patient was recruited on February 29, 2024; results are expected in the first quarter of 2026.
ConclusionsThe ANTES B+ study is the first to: (1) explore the efficacy and safety of triple therapy in a population of B+ COPD patients and (2) use a composite index (CC) as the primary result of a COPD trial.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2024 document recommends initial treatment with dual long-acting bronchodilator therapy (long-acting β2 adrenergic (LABA) and long-acting muscarinic antagonist (LAMA)) in patients with chronic obstructive pulmonary disease (COPD) group B, as defined by a COPD Assessment Test (CAT) ≥10 and none or one moderate exacerbation (treated with oral corticosteroids and/or antibiotics) in the previous year.1 Three potential caveats, however, need to be considered here. First, recent research has shown that GOLD B patients with one previous moderate ECOPD have a higher hazard ratio of future exacerbation, all-cause and respiratory hospitalization than those without it.2 Second, it is known that patients who remain symptomatic (CAT≥10) despite treatment with LABA/LAMA are at higher risk of worse health outcomes.1 And third, it is now well recognized that a higher level of circulating eosinophils (Eos) identifies patients at increased risk of future ECOPD, a better preventive response to inhaled corticosteroids (ICS), and reduced risk of ICS-related pneumonia.3 Collectively, these observations call for reconsideration of the potential role of triple therapy (LABA/LAMA/ICS) in some B patients (from now on termed B+ patients) who may be at increased risk of poor clinical control and disease progression if treated with LABA/LAMA only. Specifically, we propose that B+ patients would be characterized by the coexistence of the following three phenotypic traits: (1) remain symptomatic (CAT≥10) despite current treatment with LABA/LAMA; (2) have suffered one moderate ECOPD the previous year; and (3) have a blood eosinophil count (BEC) ≥150cells/ml. We hypothesize that triple therapy will result in better disease control and prevent disease progression than dual bronchodilator treatment in B+ patients.
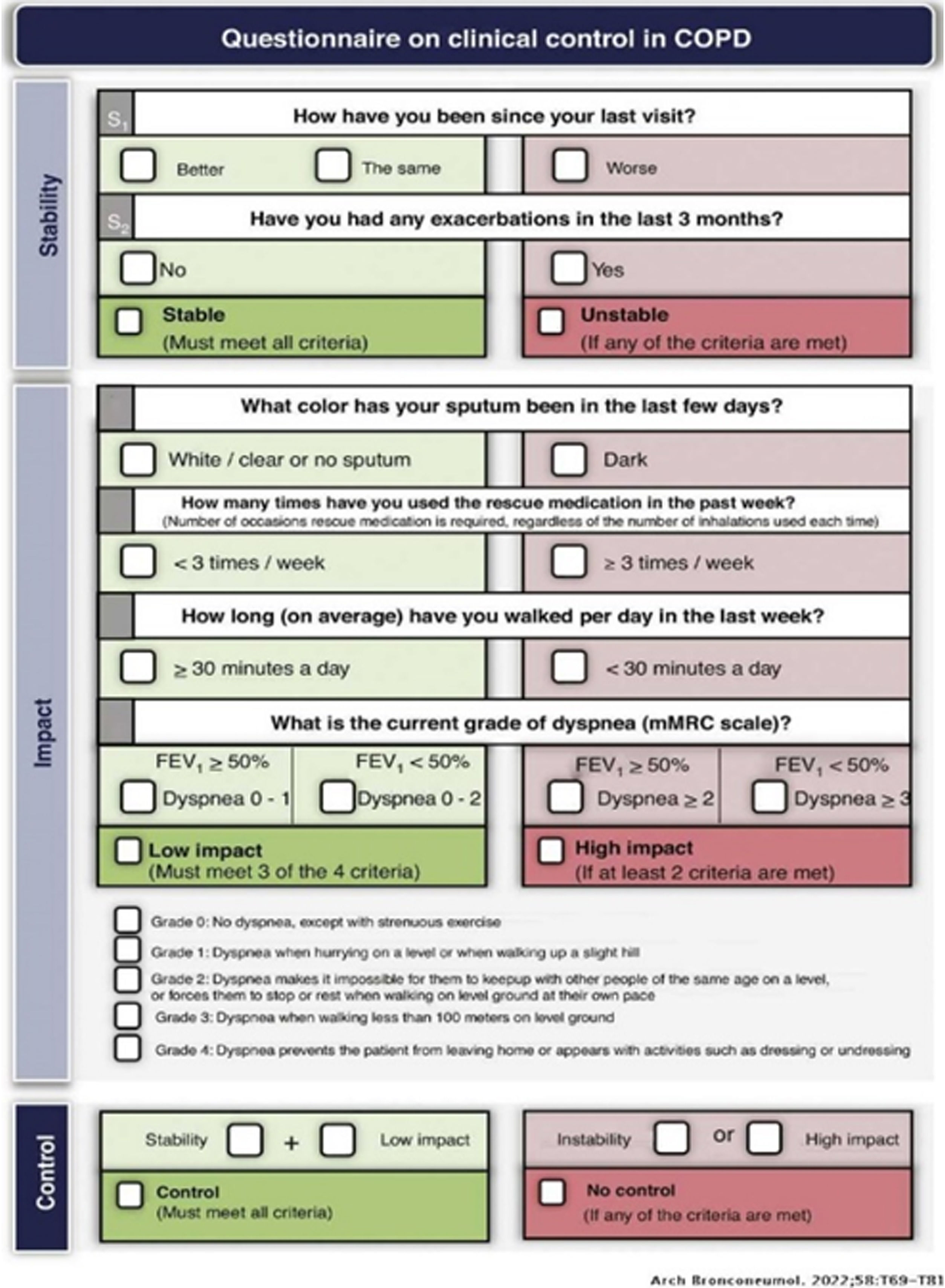
On the other hand, historically the main outcomes of randomized clinical trials (RCT) in patients with COPD have been either the level of symptoms (using a variety of questionnaires), lung function changes, the annual rate of ECOPD and/or mortality.4 However, a recent consensus document for COPD pharmacological RCTs concludes that “… the use of the new tools, particularly combination endpoints, could help better identify the right patients to be treated with the new drugs”.5 So far, however, no previous COPD RCT have used a combined endpoint as its primary outcome, at variance with cardiovascular RCTs that frequently use them (e.g., major adverse cardiovascular events – MACE).6 There are several combined endpoints that can be potentially used in RCTs in COPD,5 including the clinically important deterioration (CID),7 early clinically important improvement,8 COPDCompEx9 and clinical control (CC).10–17 As shown in Fig. 1, the latter includes two disease dimensions (impact and stability); a patient is considered “clinically controlled” if both the stability criteria and 3 out of 4 of the impact criteria are met. CC has been validated in several national and international studies that demonstrate that it predicts the risk of future exacerbations and the time to the next exacerbation.10–17 But the ANTES B+ study presented below will be the first RCTs to use a composite outcome (CC) to investigate the efficacy of a pharmacological intervention in COPD. This study has been conceived and designed by the Scientific Committee of the ANTES program, a multicenter program sponsored by GSK in Spain that aims at anticipating the diagnosis and treatment of COPD by: (1) improving COPD underdiagnosis; (2) acting earlier in younger patients; (3) early therapeutic optimization; (4) achieving an exacerbation zero goal; and (5) improve survival.18,19 Specifically, the ANTES B+ study is a collaborative study between the ANTES academic investigators in Spain and GSK aimed at testing the hypothesis that the use of triple therapy in a single inhaler (fluticasone furoate (FF) 92μg/umeclidinium (UMEC) 55μg/vilanterol (VI) 22μg) improves CC during 1 year follow-up in a larger proportion of B+ patients vs. the currently recommended dual bronchodilator treatment, as prescribed by the attending physician of the patient (LABA/LAMA).1 Here, we present the main aspects of the study protocol and discuss their novelty and potential relevance.
Domains of the clinical control (CC) composite outcome. For further explanations, see text.
Reproduced with permission from Ref. 12.
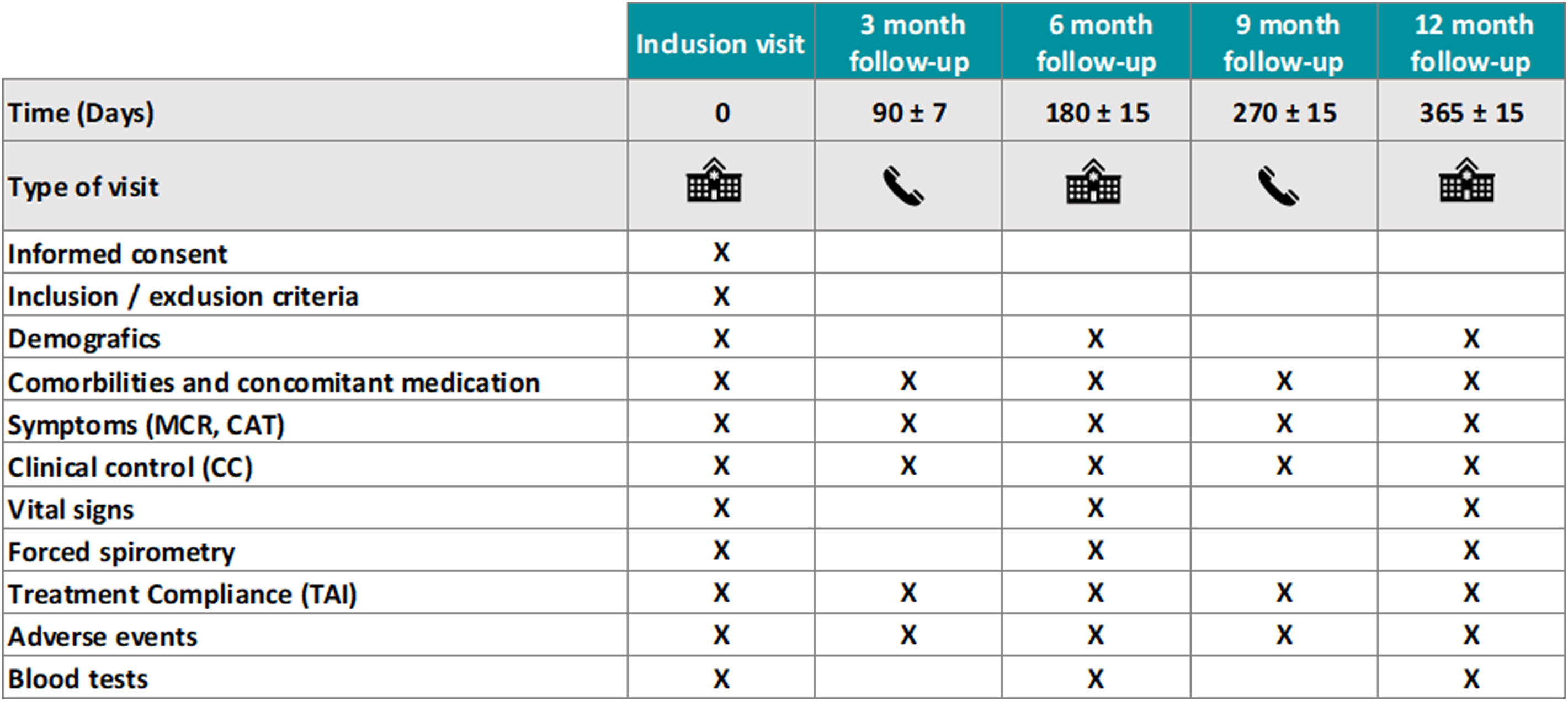
The ANTES B+ study is a prospective, multicenter, open label, randomized, pragmatic controlled pilot study in B+ COPD patients (ClinicalTrials.gov NCT06282861). Patients will be characterized at recruitment and then randomized in a 1:1 ratio to continue with their usual LABA/LAMA combination (as prescribed by their attending physician, hence a pragmatic design) or fluticasone furoate (FF) 92μg/umeclidinium (UMEC) 55μg/vilanterol (VI) 22μg in a single inhaler q.d. for 12 months. To minimize the effect of potential imbalance in the number of patients across centers, patients will be stratified per site. Likewise, randomization will be stratified per site for BEC (between 150 and 300Eos/mL and more than 300Eos/mL) to facilitate post hoc group comparisons. Then patients will be visited in the participating centers (all in Spain, both in primary and specialized care) at 6 and 12 months and contacted by phone at 3 and 9 months. The first patient was included in the study on February 29, 2024, and results are expected in the first quarter of 2026.
EthicsThe study protocol has been approved by the Ethics Committee of The Balearic Islands (Mallorca, Spain) and the Spanish National Agency for Drugs and Treatments (AEMPS). All patients will provide written informed consent before any study procedures are performed. This trial will be conducted in accordance with the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines, Good Clinical Practice guidelines as well as with all the appropriate Spanish and European regulations for biomedical research.
ObjectivesPrimaryTo determine the efficacy of FF/UMEC/VI in a single inhaler q.d. vs. the LABA/LAMA combination the patient was already receiving (as prescribed by their attending physician) in achieving a better CC in COPD B+ patients, a composite outcome that considers both impact and disease stability (Fig. 1), over a year follow-up.
SecondaryTo determine the efficacy of FF/UMEC/VI in a single inhaler q.d. vs. the LABA/LAMA combination the patient was already receiving (as prescribed by their attending physician) in COPD B+ patients on: (1) CID (using the CAT questionnaire), another validated composite endpoint8 in COPD not used as the primary outcome in any previous COPD RCT; (2) annual rate of exacerbations and FEV1 changes; and (3) the proportion of permanently vs. intermittently vs. never CC status over the study period.
ExploratoryThe following exploratory objectives will be analyzed in COPD B+ patients: (1) the primary and secondary endpoints discussed above in patients stratified by BEC (150–300Eos/mL or >300Eos/mL) or smoking status (current vs. former); (2) comparison between CC and CID-CAT results; (3) all-cause mortality between the two study arms; and (4) proportion of patients on LABA/LAMA arm that are switched to triple therapy (or vice versa), as per their physician's prescription.
Safety AnalysesThe annual incidence of adverse events and severe adverse events will be compared in both groups, including the frequency of pneumonia between the two groups.
Study PopulationThe inclusion and exclusion criteria of the study population are listed in Table 1. Patients will be recruited from Hospitals and Primary Care centers around Spain.
Trial Population of the B+ Study.
| Inclusion criteria |
| • Female or male. |
| • 40–80 yrs. of age. |
| • Current/former smokers ≥10 pack-year. |
| • Diagnosis of COPD according to GOLD 2024 (post-BD FEV1/FVC <0.7 in the appropriate clinical context) with FEV1 post-BD 30–70% of the reference value. |
| • B+ phenotype. |
| ∘ CAT ≥10 despite being on LABA/LAMA for ≥3 months. |
| ∘ 1 moderate ECOPD in the previous year (treated with a short course of oral steroids and/or antibiotics). |
| ∘ ≥150 blood Eos/mL (as determined by a single Eos measurement in the previous 12 months available in the medical record of the patient). |
| • A signed and dated written informed consent prior to study participation. |
| Exclusion criteria |
| • GOLD E (≥2 moderate or 1 severe ECOPD in the previous year). |
| • ICS treatment (or oral steroid for whatever reason) during the last 8 weeks.22 |
| • ECOPD during the last 8 weeks.22 |
| • Current diagnosis of asthma or documented history of asthma in the medical record of the patient according to the 2023 Global Initiative for Asthma (GINA) guidelines or other accepted guidelines. |
| • Other concomitant respiratory disease (e.g., bronchiectasis, lung fibrosis, lung neoplasm). |
| • Use of domiciliary long-term oxygen therapy or non-invasive ventilation. |
| • Alpha-1 antitrypsin deficiency. |
| • Unstable or life-threatening cardiac disease, including: |
| • Myocardial infarction or unstable angina in the last 6 months. |
| • Unstable or life-threatening cardiac arrhythmia requiring intervention in the last 3 months. |
| • NYHA class IV heart failure. |
| • Starting a Pulmonary Rehabilitation Program within the 4 weeks prior to screening, or planning to do so during the study. |
| • Long-term antibiotic therapy (antibiotics are allowed for the short-term treatment of an exacerbation or for short-term treatment of other acute infections during the study). |
| • Systemic, oral, parenteral corticosteroids used for COPD and/or other diseases in the 8 weeks before entering in the study (oral/systemic corticosteroids may be used to treat COPD exacerbations during the study). |
| • Active neoplasm. |
| • Life expectancy <1 yr. |
| • Current participation in other RCTs. |
| • Non-compliance: subjects at risk of non-compliance, or unable to comply with the study procedures. |
| • Any disease, disability, or geographic location that would limit compliance for scheduled visits. |
| • Known allergy to FF/UMEC/VI q.d. components (vilanterol, umeclidinium and/or fluticasone furoate) or inability to use the Ellipta® device. |
| • Women who are pregnant or lactating or are planning to become pregnant during the study. |
Fig. 2 summarizes all trial assessment and procedures throughout the study. Patient compliance with the trial intervention will be monitored in each of the follow-up visits through the Test of Adherence to Inhalers (TAI) questionnaire.20 Concomitant therapy for potential comorbidities will be allowed as per the attending physician and recorded appropriately. Short-acting bronchodilators as rescue therapy will be allowed (and recorded as measure of “clinical control” (Fig. 1)). The Barcelona Clinical Coordinating Center (https://bcccbarcelona.com/en/) of the Private Foundation Mon Clínic Barcelona will provide support for the study as an academic Contract Research Organization.
Statistical AnalysisSample Size DeterminationThere is no published data available in the study population of COPD B+ patients that allows a precise estimation of the sample size for the ANTES B+ trial. Accordingly, we used data published in other, generally more severe, COPD populations21–23 to estimate it. According to these previous reports, we hypothesized that: (1) 30% of B+ patients treated with LABA/LAMA will be CC at all clinical visits during the study duration (permanent CC) and (2) that FF/UMEC/VI q.d. will improve the number of CC patients by 9% (up to 39%). Based on these calculations, the expected odds ratio (OR) to be detected is 1.49. Thus, for a bilateral test, with α-risk of 0.05, and a β-risk of 0.2 (80% power), assuming that a 15% of patients can be lost during follow-up, we estimated that the study needs to recruit a total of 1028 patients, and that 514 patients need to be randomized to each arm of the study (LABA/LAMA or FF/UMEC/VI q.d.).
Data AnalysisStandard descriptive statistics (n, range, %, mean±standard deviation, median [interquartile range−IQR]) and unpaired T-test for continuous normally distributed parameters, chi-square test to compare qualitative variables or the Wilcoxon rank-sum test for continuous parameters not normally distributed will be used to compare the main characteristics of both groups at recruitment, in order to ensure that the two arms are comparable at baseline. The analysis of the primary and secondary objectives will be based on a two-sided univariate analysis and several multivariate adjusted models. Endpoints involving time to first event will be assessed by using Kaplan–Meier models, compared by log-rank tests, with univariate and multivariate Cox regression models (covariates including but not limited to age, sex, FEV1, smoking). For exploratory objectives, correlations between dimensions and variables that forms each tool Correlations index (Pearson's correlation coefficient or Cohen's kappa coefficient) will be used. The combination of Kaplan–Meier models, compared by log-rank tests, with univariate and multivariate logistic regression models, Cox regression models will be used to assess all-cause mortality. Data analysis will be conducted using R and SPSS.
DiscussionThe working hypothesis of the ANTES B+ study is that triple therapy improves a novel composite health outcome (CC) in a particular subgroup of GOLD B patients (B+ patients). No previous study has investigated this hypothesis before, so the ANTES B+ study is exploring new territory. However, some recent previous studies in different patient populations provide the rationale to test it. First, the E-max study by Maltais et al. showed that a LABA–LAMA combination was more effective in terms of symptom improvement (transitional dyspnea index) than any of the two bronchodilators alone22; in fact, this forms the basis for the GOLD recommendation of a LABA–LAMA combination as starting treatment in B patients.1 Second, in a short study (12 weeks), Han et al. failed to show that dual bronchodilator treatment decrease respiratory symptoms in symptomatic, tobacco-exposed persons with preserved lung function as assessed by spirometry, although it improved significantly after treatment.24 Third, two large RCTs (IMPACT21 and ETHOS23) showed that, in more severe patients, triple therapy reduces all-cause mortality after one year follow-up; indeed, this is the main reason why GOLD considers the use of triple therapy as initial treatment in GOLD E patients with >300Eos/μL.1 And, finally, it is now well accepted that the level of circulating eosinophils identifies a group of patients at risk of exacerbations who may respond well to treatment with ICS.3 Because B+ patients have never been studied in isolation, we do not know how Eos levels can inform treatment decisions (dual vs. triple therapy) in B+ patients, but the results of the ANTES B+ study will provide relevant information in this setting.
Another important aspect to note, is that the ANTES B+ study will be the first RCT to use a composite outcome (CC) as the primary endpoint in COPD. CC has been validated in several national and international studies that demonstrate that it predicts the risk of future exacerbations and the time to the next exacerbation,10–17 but never before as a measure of the efficacy of a therapeutic intervention. This is perfectly in line with the recently revisited ATS/ERS recommendations on outcomes for COPD pharmacologic trials.5
It is also of note that the ANTES B+ study takes a pragmatic approach (the control group will continue to be treated with their LABA/LAMA combination, as prescribed before entering the study by their attending physician), yet robust (1 year-long follow-up using a novel primary composite outcome) to answer a key clinical question in a study population at risk of poor CC. The final strategic goal of the study would be to inform guidelines and to be relevant to prescribing physicians.
The design of the ANTES B+ study is aligned with the strategic goals of the ANTES program, in particular those of early therapeutic optimization, zero exacerbations and, hopefully, improved survival.18,19 So far, recommendations for pharmacologic treatment of COPD lagged behind the disease: more symptoms, more treatment; more exacerbations, more treatment.1 If the ANTES B+ study is positive, this paradigm will have to be revisited to optimize their pharmacological treatment earlier in the course of the disease in B+ patients.
FundingThe ANTES B+ study is a collaborative study between the academic members of the ANTES program and GSK (study number #221775).
Authors’ ContributionsAA wrote the first draft of the paper. All authors read it and contributed comments to it. The final manuscript was approved by all authors.
Competing InterestsThe authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Agusti A: declares research support, lecture fees and participation in advisory board in AstraZeneca, Chiesi, GSK, Gebro, Menarini, MSD, Sanofi-Regeneron and/or Zambon. He does not have shares or interest in any company, neither does any member of his family. He has not received or had any relationship with tobacco money.
Lopez-Campos JL has received honoraria during the last 3 years for lecturing, scientific advice, participation in clinical studies or writing for publications for (alphabetical order): AstraZeneca, Bial, Boehringer, Chiesi, CSL Behring, Faes, Gebro, Grifols, GSK, Menarini, Zambon.
Miravitlles M: declares speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Glenmark Pharmaceuticals, Menarini, Kamada, Takeda, Zambon, CSL Behring, Specialty Therapeutics, Janssen, Grifols and Novartis, consulting fees from AstraZeneca, Atriva Therapeutics, Boehringer Ingelheim, BEAM Therapeutics, Chiesi, GlaxoSmithKline, CSL Behring, Ferrer, Inhbrix, Menarini, Mereo Biopharma, Spin Therapeutics, Specialty Therapeutics, ONO Pharma, Palobiofarma SL, Takeda, Novartis, Novo Nordisk, Sanofi/Regeneron, Zambon, Zentiva and Grifols and research grants from Grifols.
Soler-Cataluña JJ declares grant research from GlaxoSmithKline, speaker fees from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, and Menarini, and consulting fees from Bial, Chiesi, and GlaxoSmithKline.
Marin JM declares to have received unrestrictive grants from AstraZeneca and GSK and participation in advisory boards for GSK, and Sanofi-Regeneron.
Cosio BG declares grants from Chiesi and GSK; personal fees for advisory board activities from Chiesi, GSK, Novartis, Sanofi, Teva, and AstraZeneca; and payment for lectures/speaking engagements from Chiesi, Novartis, GSK, Menarini, AstraZeneca, and Sanofi outside the submitted work.
Alcázar-Navarrete B reports grants and personal fees from GSK, personal fees and non-financial support from Boehringer Ingelheim, personal fees and non-financial support from Chiesi, non-financial support from Laboratorios Menarini, grants, personal fees and non-financial support from AstraZeneca, personal fees from Gilead, personal fees and non-financial support from MSD, personal fees from Laboratorios BIAL, personal fees from Zambon, outside the submitted work.
Echave-Sustaeta JM has received speaker or consulting fees from GlaxoSmithKline in the last 3 years.
Casanova C has received speaker or consulting fees from AstraZeneca, GlaxoSmithKline, Menarini, Novartis, and research grants from GlaxoSmithKline, Menarini and AstraZeneca in the last 3 years.
Peces-Barba G declares grants from GSK, Chiesi and Menarini.
De-Torres JP declare lecture fees and participation in advisory boards for GSK, AstraZeneca, Sanofi-Regeneron.
Fernandez-Villar A declares research support, lecture fees and participation in advisory board in AstraZeneca, Chiesi, Grifols and GSK.
Ancochea J declares lecture fees and collaboration or participation in advisory boards for Air Liquide, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, Ferrer, GSK, Linde Healthcare, Menarini, Mundipharma, Novartis, Roche, Rovi, Sandoz and Teva.
Villar F has attended or participated in activities organized or financed by the pharmaceutical companies Almirall, AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Esteve, Ferrer, Menarini, Novartis, Mundipharma, Orion, Pfizer, Teva and Zambon.
Roman-Rodriguez M declares grants from AZ; personal fees for advisory board activities from GSK and AstraZeneca; and payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Cipla, Chiesi, Faes, Gebro, GlaxoSmithKline, Menarini Pfizer, and Sanofi outside the submitted work.
Molina J declares lecture fees and participation in advisory board in AstraZeneca, Chiesi, GSK, and Menarini.
Garcia-Rivero JL reports speaker fees from Gebro Pharma, GSK, AstraZeneca, Chiesi, Menarini and Sanofi; consulting fees from GSK, AstraZeneca, ALK, and Sanofi.
Gonzalez C declares research support, lecture fees and participation in advisory board in AstraZeneca, CLS Behring, Grifols, BIAL, Zambon and GSK.
Sobradillo P has received honoraria during the last 3 years for lecturing, scientific advice, participation in clinical studies or writing for publications for (alphabetical order): AstraZeneca, Bial, Boehringer, Chiesi, CSL Behring, Faes, Gebro, Grifols, GSK.
Faner R declares research support, lecture fees or participation in advisory boards for AstraZeneca, Chiesi, GSK, Menarini, Sanofi-Regeneron and/or Zambon.
Peña C is a full-time GSK employee.
Sharma R is a full-time GSK employee.
Celli B reports having received compensation for Advisory Boards and consultation fees from: GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, Novartis, Pulmonx, Sanofi-Aventis, CHIESI, Menarini and Gala Therapeutics. He does not have shares or interest in any company, neither does any member of his family. He has not received or had any relationship with tobacco money.
Authors thank the Barcelona Clinical Coordinating Center of the Private Foundation Mon Clinic in Barcelona (Spain) (https://bcccbarcelona.com/en), and particularly Lada Murcia (PhD), for their help and outstanding support to develop the study protocol, the eCRF to be used in the study and all the administrative and logistic issues involved in a large, multicenter RCT like the ANTES B+ study. Authors would also like to acknowledge and thank Almudena Blanco (GSK) for her energy, enthusiasm and support in the ANTES program.