Recent research has shown that chronic obstructive pulmonary disease (COPD) may originate early in life,1–3 and that suboptimal lung function is associated with numerous factors that interact and accumulate over time4 (Fig. 1). These findings have opened new windows of opportunity for diagnosis and early therapeutic intervention in COPD.5 The ANTES program (Anticipating the Diagnosis and Treatment of COPD in the 21st Century) funded by GlaxoSmithKline (GSK) and supervised by a scientific committee of independent experts5 was launched nearly 1 year ago to pursue the following objectives: (1) reduce the underdiagnosis of COPD, (2) take action at an early stage of the disease, (3) optimize therapeutic interventions from the start of treatment, (4) avoid COPD exacerbations, and (5) improve the prognosis of COPD patients.5 The research projects started since the launch of the program are summarized below.
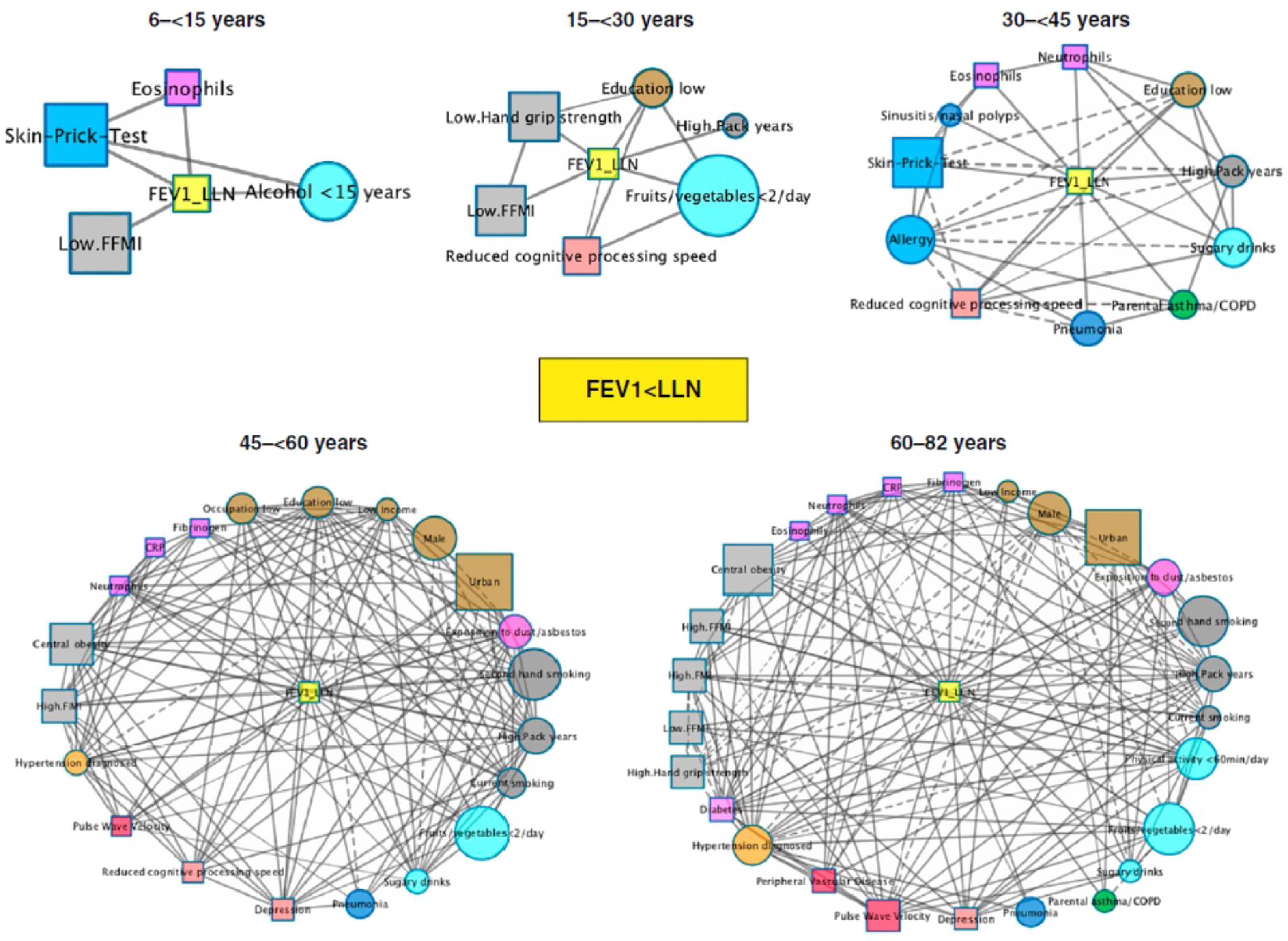
First-neighbor networks for FEV1<lower limit of normal (LLN) (center yellow node) in different age bins of the LEAD cohort (Austria). The size of each node is proportional to its prevalence in LEAD in that age bin. The color of the node indicates the type of variable. The connections between nodes indicate the existence of significant correlations (p<0.05) between them and the type of line indicates whether the OR is greater than (solid) or less than 1 (dashed). Reproduced from Breyer-Kohansal, et al.4 with permission of the Am J Respir Crit Care Med.
The aim of this project is to develop and validate a mobile app that uses the smartphone microphone to estimate our level of respiratory health. A campaign was launched on social networks asking users to try the app (RespirActúa) and earn points for performing maneuvers similar to those used in spirometry tests. This allowed researchers to collect data from more than 3000 maneuvers, and showed that the app is capable of capturing the biological signal and differentiating between maneuvers performed correctly and incorrectly. Over the next few months, this technology will be validated against spirometry tests performed in a lung function laboratory. It is important to point out that this app is not intended to replace traditional spirometry tests, but rather to provide an easily accessible indicator of lung health that can advise users to contact the health services for a more accurate lung function test if any abnormalities are detected.
Exploring the origins: the MaterANTES studyIn a healthy individual, the lungs develop and mature from birth, during childhood and adolescence, and reach their peak function at around 20–25 years of age, or slightly earlier in women.6 Due to various genetic and environmental factors (Fig. 1), this developmental process is suboptimal in 4–12% of the general population, who never reach peak normal lung function.1,2,7,8 Recent evidence has shown that young adults with low lung function develop a greater number of morbidities6 and have a shorter life span.9 Prematurity compromises the development of the airway and lung parenchyma, and increases the incidence of respiratory comorbidities in adulthood.9 Even as toddlers, these children present more wheezing, hospitalizations, and functional deficit, with low FEV1 and FVC.10 This functional deterioration seems to persist in the final stage of growth, and is also associated with a higher prevalence of pulmonary symptoms that require treatment.11 The same appears to be true of at-term infants with very low birth weight.12 Early diagnosis and treatment of this subgroup of adults who were born prematurely or with low birth weight is therefore of potential clinical relevance.5 However, no studies have yet explored the effects of existing therapies in these individuals, and it would be of considerable interest to create a cohort of young subjects with suboptimal lung function secondary to prenatal and perinatal factors that can be used in future studies to determine the effect of a potential therapeutic intervention. This type of cohort can easily be extracted from records held by the country's main maternity hospitals since the early 1980s. The MaterANTES project is a pilot study that explores the feasibility of creating a cohort of individuals aged between 20 and 50 years with poor lung function (FEV1<80% predicted) associated with perinatal factors (prematurity, low weight) that can be studied to evaluate and eventually treat the effect of these factors on lung function in young adults.
Investigating the young COPD population in EPISCAN IIThe EPISCAN II study, recently published in Archivos de Bronconeumología,13 evaluated the spirometry tests of 9092 volunteers (general population) over 40 years of age in all 17 Spanish autonomous communities.10 Mean age was 60±11 years; 52.6% were men. The cohort included 1900 subjects aged between 40 and 50 years, 4.1% of whom met spirometric criteria for COPD, although only 9% had already been diagnosed. This shows that the underdiagnosis of COPD – 74.7% in the population aged over 50 years – is even more widespread in individuals under 50 years of age. Of note, slightly over a quarter (28.2%) of these young patients had previously been diagnosed with asthma. It is interesting to note that young COPD patients have a higher symptom burden, worse CAT score, and a higher frequency of exacerbations than other age groups.10 Studies are currently under way in subjects who would meet the criteria of the proposed pre-COPD category, i.e., individuals with respiratory symptoms or pulmonary emphysema on computed tomography or a diffusing capacity for carbon monoxide (DLCO)<80% predicted and non-obstructive spirometry.11
GOLD 0, DLCO 1: a look beyond the obstructionThis project (www.clinicaltrials.govNCT04409275) is based on the hypothesis that exclusive reliance on spirometry in the diagnosis of COPD may underestimate existing, clinically relevant physiological impairment. The researchers suggest that DLCO determination may provide relevant information in smokers with normal spirometry, and have therefore designed a multicenter prospective observational study in 20 Spanish hospitals that will recruit smokers or former smokers with ≥10 cumulative pack-years who present respiratory symptoms but have normal spirometric results and normal or abnormal DLCO (<80% predicted). Both groups will be followed prospectively for 5 years to assess the effect of these physiological changes on clinically relevant outcomes. The researchers will also analyze serum markers of oxidative stress, inflammation and tissue repair, and changes from baseline in high-resolution computed tomography images. The project is expected to provide relevant information on the natural history of this disease in patients with no airflow obstruction14 who are now categorized as pre-COPD.11
New definition of COPD exacerbationExacerbation is a key element in the natural history of COPD due to the significant impact these episodes have on the health and prognosis of these patients.15 However, COPD exacerbation is currently defined solely as a worsening of the patient's respiratory symptoms, and as such is non-specific.15 Some alternative definitions based on the measurement of various biomarkers have recently emerged,16 followed by a new disease-focused definition (the Rome proposal),17 although the Spanish COPD guidelines (GesEPOC) are in favor of a new syndromic approach that focusses more on the characteristics of the patient than on the exacerbation event.18 The ANTES program is currently exploring the possibility of conducting a prospective, national, multicenter, multilevel study (primary and specialized care) to explore these alternatives.
ConclusionsOne year after its launch,5 the ANTES program is in full swing. In line with the initial aims of the program, the authors of a recently published special article have emphasized the importance of performing interventional clinical trials in young patients with COPD or pre-COPD.19 COPD is still a largely misunderstood disease, and the COVID-19 pandemic has dramatically highlighted the importance of lung health.20 The ANTES program aims not only to improve the diagnosis and treatment of COPD through earlier diagnostic and therapeutic interventions, but also to raise awareness of the importance of preserving respiratory health and promoting healthy ageing.5
FundingThe ANTES program is funded by GSK.
Conflict of interestsMarc Miravitlles has received speaker's fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Bial, Sandoz, Zambon, CSL Behring, Grifols and Novartis; consulting fees from AstraZeneca, Atriva Therapeutics, Boehringer Ingelheim, Chiesi, Ferrer, GlaxoSmithKline, Bial, Gebro Pharma, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, Spin Therapeutics, ONO Pharma, pH Pharma, Palobioframa SL, Novartis, Sanofi and Grifols; and research grants from Grifols. José María Marín has received speaker's and consultancy fees over the past two years from Chiesi, Menarini and GlaxoSmithKline and research grants from GlaxoSmithKline, Menarini and AstraZeneca. José Luis López-Campos has received speaker's and consultancy fees over the past 3 years and has participated in clinical trials and written publications for (in alphabetical order): AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, CSL Behring, Ferrer, Gebro, GlaxoSmithKline, Grifols, Menarini, Megalabs, Novartis, and Rovi.
The scientific committee of the ANTES program would like to thank GSK for its financial and logistic support of the projects presented in this manuscript.