This article details the GesEPOC 2021 recommendations on the diagnosis and treatment of COPD exacerbation syndrome (CES). The guidelines propose a definition-based syndromic approach, a new classification of severity, and the recognition of different treatable traits (TT), representing a new step toward personalized medicine. The evidence is evaluated using GRADE methodology, with the incorporation of 6 new PICO questions. The diagnostic process comprises four stages: 1) establish a diagnosis of CES, 2) assess the severity of the episode, 3) identify the trigger, and 4) address TTs. This diagnostic process differentiates an outpatient approach, that recommends the inclusion of a basic battery of tests, from a more comprehensive hospital approach, that includes the study of different biomarkers and imaging tests. Bronchodilator treatment for immediate relief of symptoms is considered essential for all patients, while the use of antibiotics, systemic corticosteroids, oxygen therapy, and assisted ventilation and the treatment of comorbidities will vary depending on severity and possible TTs. The use of antibiotics will be indicated particularly if sputum color changes, when ventilatory assistance is required, in cases involving pneumonia, and in patients with elevated C-reactive protein (≥ 20 mg/L). Systemic corticosteroids are recommended in CES that requires admission and are suggested in moderate CES. These drugs are more effective in patients with blood eosinophil counts ≥ 300 cells/mm3. Acute-phase non-invasive mechanical ventilation is specified primarily for patients with CES who develop respiratory acidosis despite initial treatment.
En este artículo se presentan las recomendaciones sobre el diagnóstico y tratamiento del síndrome de agudización de la enfermedad pulmonar obstructiva crónica (EPOC) (SAE) de GesEPOC 2021. Como principales novedades, la guía propone una definición y aproximación sindrómica, una nueva clasificación de gravedad y el reconocimiento de diferentes rasgos tratables (RT), lo que supone un nuevo paso hacia la medicina personalizada. La evaluación de la evidencia se realiza mediante la metodología Grading of Recommendations Assessment, Development and Evaluation (GRADE), con la incorporación de seis nuevas preguntas con enfoque paciente, intervención, comparación y resultados (PICO). El proceso diagnóstico comprende cuatro etapas: 1) establecer el diagnóstico del SAE, 2) valorar la gravedad del episodio, 3) identificar el factor desencadenante y 4) abordar los RT. En este proceso diagnóstico se diferencia una aproximación ambulatoria, en la que se recomienda incluir una batería básica de pruebas y una hospitalaria, más exhaustiva, en la que se contempla el estudio de diferentes biomarcadores y pruebas de imagen. El tratamiento broncodilatador destinado al alivio inmediato de los síntomas se considera esencial para todos los pacientes, mientras que el uso de antibióticos, corticoides sistémicos, oxigenoterapia, ventilación asistida o el tratamiento de las comorbilidades variará en función de la gravedad y de los posibles RT. El empleo de antibióticos estará especialmente indicado ante un cambio en el color del esputo, cuando se requiera asistencia ventilatoria, en los casos que cursen con neumonía y también para aquellos con proteína-C reactiva elevada (≥ 20 mg/L). Los corticoides sistémicos se recomiendan en el SAE que necesita ingreso y se sugieren en el SAE moderado. La eficacia de estos fármacos es mayor en pacientes con recuento de eosinófilos en sangre ≥ 300 células/mm3. La ventilación mecánica no invasiva en fase aguda se establece fundamentalmente para pacientes con SAE que cursen con acidosis respiratoria, a pesar del tratamiento inicial.
Four years have now passed since the publication in 2017 of the last Spanish Chronic Obstructive Pulmonary Disease Guidelines (GesEPOC), which included a specific section on exacerbation of the disease.1 Since then, a number of scientific advances have been made that require these recommendations to be updated.2•4 The current recognition of exacerbation as a syndrome resulting from different etiopathogenic mechanisms, all with similar clinical expression, and the ongoing need to move toward increasingly personalized medicine2,5 have prompted the GesEPOC scientific committee to update its recommendations, including the latest evidence and some new proposals, such as a definition-based syndromic approach, classification of severity and the identification of different treatable traits (TT).
In this paper, we present recommendations on the diagnosis and treatment of chronic obstructive pulmonary disease (COPD) exacerbation syndrome (CES) in the new GesEPOC 2021. Representatives of the scientific societies involved in the treatment of patients with COPD and the Spanish Patient Forum have participated in drawing up the guidelines. These have been developed following the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology.6 In this edition of the guidelines, 6 new PICO (patient, intervention, comparison and outcomes) questions have been formulated. Details of the protocol, including the PICO questions, literature search and evidence tables can be found in the supplementary material. Table 1 lists the key points of this update.
Key points.
| COPD exacerbation is considered a syndrome resulting from different etiopathogenic mechanisms, all of which have a similar clinical expression. |
| In circumstances where it is difficult to discriminate whether clinical worsening is a result of COPD or a comorbidity, both processes should be diagnosed and treated. |
| Pneumonia is included within CES. |
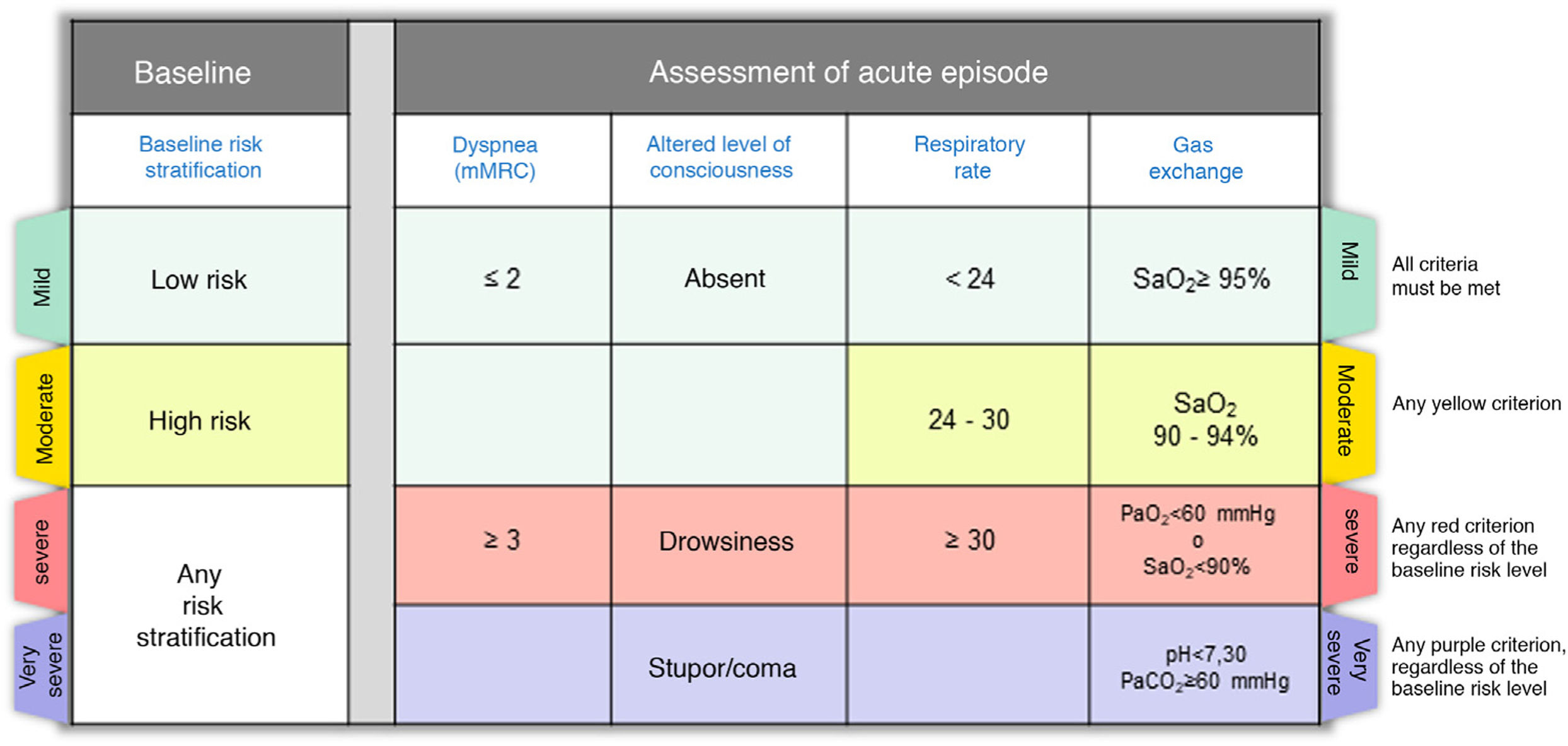
| The severity of the CES should be assessed according to the baseline risk stratification (low or high risk) and the severity of the acute episode (level of dyspnea, level of consciousness, respiratory rate and gas exchange). |
| The treatment of choice for CES is short-acting and rapid-acting bronchodilators. |
| Systemic corticosteroids are suggested for moderate CES and recommended for all patients with severe or very severe CES. Their efficacy is greater when the peripheral blood eosinophil count is ≥ 300 cells/mm3 |
| Other treatment should be guided by severity level and the identification of different treatable traits. |
CES: chronic obstructive pulmonary disease exacerbation syndrome; COPD: chronic obstructive pulmonary disease.
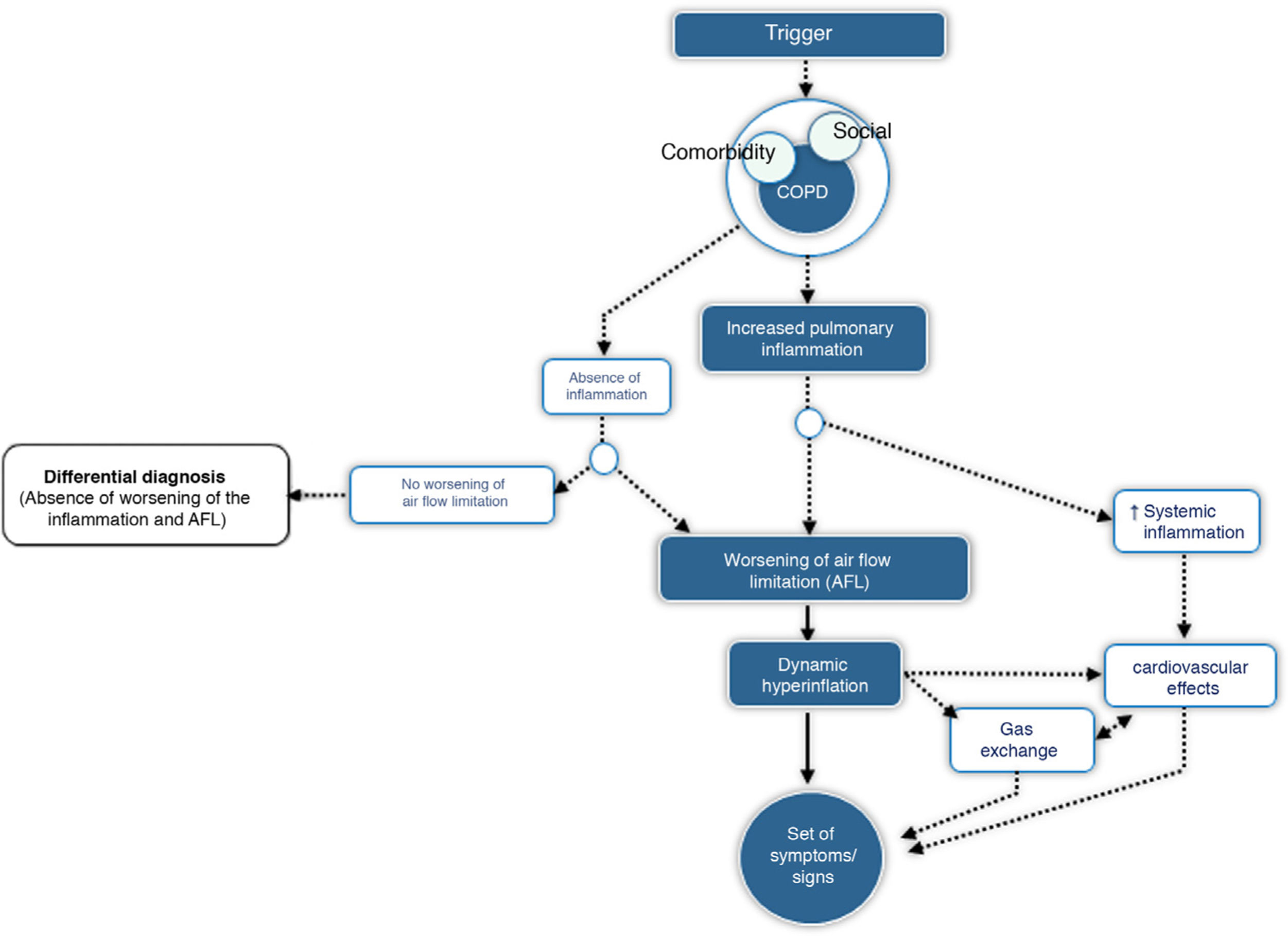
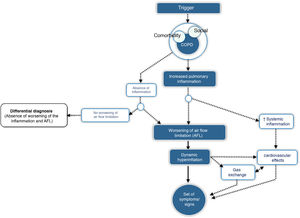
CES is defined as an episode of clinical instability that occurs in a patient with COPD as a result of worsening of expiratory airflow limitation or the underlying inflammatory process, and is characterized by an acute worsening of the individual's respiratory symptoms relative to baseline. From a pathophysiological point of view, CES is a complex, heterogeneous event comprising a diverse set of alterations, which are either isolated or, more frequently, combined and clinically expressed in a similar manner in the COPD patient. Fig. 1 outlines the core pathophysiological elements of CES.
Many CES occur in clusters, raising the question of whether they are actually new events or incomplete resolutions of previous episodes. To distinguish between these situations, the following definitions have been established1:
Treatment failure: when worsening of symptoms occurs during the CES itself and requires additional treatment.7 Average recovery time after CES is approximately 2 weeks. However, some patients do not fully recover until 4-6 weeks.8,9
Relapse: when further worsening of symptoms occurs between the end of CES treatment and the next 4 weeks.
Recurrence: when symptoms reappear within 1 year of the preceding CES, after a period of relative good health. At least 4 weeks must have passed after completing the previous CES treatment or 6 weeks after onset of symptoms.7 Recurrences are considered new episodes of CES.
DiagnosisIn order to properly diagnose and categorize the CES, it must first be confirmed that this is an exacerbation syndrome in a patient with COPD, making the appropriate differential diagnosis beforehand. Severity should subsequently be established, trigger factors assessed, and potential TTs identified. The diagnostic approach may differ depending on whether the CES occurs in an outpatient or an inpatient.
Step 1: Diagnosis of COPD exacerbation syndromeClinical suspicion should be established when there is acute, sustained and significant worsening of respiratory symptoms (dyspnea, cough, changes in sputum color or volume) from baseline in a patient with a previous diagnosis of COPD. The cardinal symptom of CES is the significant increase in dyspnea. In order to document worsening of dyspnea, the patient's baseline status should be known and described. GesEPOC recommends the use of the modified Medical Research Council (mMRC) scale to assess the degree of dyspnea.10 Increased coughing or changes in color and/or increased volume of sputum are also considered symptoms of CES. The diagnosis should be confirmed when, in addition to the above criteria, the corresponding differential diagnosis has been made.
Differential diagnosisTable 2 shows the main diseases to be considered in the differential diagnosis. It is sometimes difficult to distinguish whether the origin of the symptoms is specific to the COPD or related to the comorbidity. In these cases, both processes should be diagnosed and treated. For example, it has been reported that between 20% and 30% of severe CES are associated with heart failure,11,12 and that between 15% and 20% of cases have myocardial damage with elevated troponin levels.13
Approximately 20% of patients with CES have parenchymal infiltrates on chest X-ray14,15 and slightly more than one third of severe cases also show them on computed tomography (CT), despite not being observed on plain chest X-ray.16 Traditionally, these infiltrates • labeled as pneumonia • have been considered a comorbidity in COPD. However, there is a very fine line between COPD exacerbation and pneumonia. The symptoms are virtually identical and the underlying mechanisms similar, so from a syndromic point of view, both conditions fall under CES. The lung microbiome does not differ among COPD patients with or without pneumonic infiltrate, and the triggers are similar, as is treatment.15 The main difference is that, in the case of pneumonia, the inflammation is greater and the prognosis worse,14,17 suggesting that these could be different expressions of the same pathological process. For this reason, pneumonia, classified in previous editions of GesEPOC as a comorbidity,1 is currently considered a type of CES.
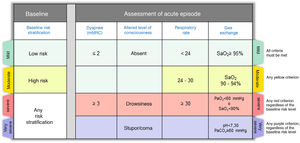
Step 2: Assess the severity of the episodeOnce the diagnosis of CES has been established, it is very important to describe the severity of the episode, which in most cases is the result of the interaction between the underlying disease and the severity of the acute episode. Fig. 2 shows the severity criteria proposed by GesEPOC. Baseline risk stratification should be performed according to the latest GesEPOC 2021 update for stable disease,4 while the severity of the episode should be evaluated according to the level of dyspnea, level of consciousness, respiratory rate and gas exchange.
CES severity criteria. Mild: all criteria shown in green must be met; moderate: if there are any yellow criteria; severe: any red criteria, regardless of baseline risk stratification; very severe: any purple criteria, regardless of baseline risk stratification.
CES: chronic obstructive pulmonary disease exacerbation syndrome.
Predictive risk scores may be of great interest when stratifying patients and designing care pathways of different intensity and/or complexity.18•23 One of the most studied scores is the DECAF index, developed by Steer et al.20 in patients hospitalized for CES from the 5 predictors of mortality with more specific weight (baseline dyspnea, eosinopenia, consolidation, acidemia and atrial fibrillation) (Table 3). This index has shown excellent discrimination for mortality and is more accurate than other clinical predictors of mortality.20•23 In low-risk patients (0 to 1 point), in-hospital mortality was 0.5% to 2.1% while 30-day mortality ranged from 1.5% to 3.8%; in intermediate-risk cases (2 points), in-hospital mortality was 8.4% while 30 day-mortality was 11.9%. Patients with DECAF ≥ 3 points had in-hospital mortality of 24% to 70% and 30 day-mortality ranging from 27.2% to 70%.17 GesEPOC recommends the additional use of predictive risk scores, such as the DECAF index, in inpatients with CES (severe or very severe).
DECAF index for patients hospitalized for CES.
| Variable | Score |
|---|---|
| Dyspnea | |
| eMRCD 5a | 1 |
| eMRCD 5b | 2 |
| Eosinopenia (<0.05 í 109/L) | 1 |
| Consolidation | 1 |
| Acidemia (pH < 7.3) | 1 |
| Atrial fibrillation | 1 |
CES: chronic obstructive pulmonary disease exacerbation syndrome; DECAF: Dyspnea, Eosinopenia, Consolidation, Acidemia, and Atrial Fibrillation; eMRCD: extended Medical Research Council dyspnea scale, where dyspnea grade 5 is equivalent to grade 4 on the mMRC scale (10); eMRCD 5 a: patients who are independent in washing or dressing; eMRCD 5 b: patient requires assistance in washing and dressing.
Taken with permission from Steer et al.20.
Table 4 lists the most common triggers for CES.24 Identifying them is of the utmost importance for proper treatment. However, in about one third of cases, the triggers are not identified. Among the best-known triggers are respiratory infections, both viral and bacterial. The criterion that best predicts bacterial infection is a change in sputum color.25 In contrast, mucoid sputum is rarely associated with bacterial infection.26 Sputum analysis (gram stain and culture) is particularly indicated in patients with severe or very severe CES who present with frequent exacerbations, need for assisted ventilation, or in the event of treatment failure. The use of molecular techniques such as reverse transcriptase polymerase chain reaction (RT-PCR) has revealed that between 22% and 64% of COPD exacerbations are viral.27 A significant proportion of viral-bacterial co-infection has also been identified.28 Recently, automated molecular diagnostic systems have been marketed that allow multiple respiratory viruses to be detected simultaneously. The cost of these tests does not yet allow their widespread use in clinical practice. Nevertheless, it is very likely that in the immediate future such techniques can be incorporated into the CES diagnostic scheme. The SARS-CoV-2 pandemic has accelerated the use of RT-PCR to screen for viruses in clinical practice.
CES triggers.
| Viruses | Rhinovirus (common cold) |
| Influenza | |
| Parainfluenza | |
| Coronavirus (including SARS-CoV-2) | |
| Adenovirus | |
| Respiratory syncytial virus | |
| Bacteria | Haemophilus influenzae |
| Streptococcus pneumoniae | |
| Moraxella catarrhalis | |
| Pseudomonas aeruginosa | |
| Atypical organisms: | |
| Chlamydophila pneumoniae | |
| Mycoplasma pneumoniae. | |
| Air pollution | Ozone (O3) |
| Suspended particulates ≤ 10 µm in diameter (PM10) | |
| Sulfur dioxide (SO2) | |
| Nitrogen dioxide (NO2) |
CES: chronic obstructive pulmonary disease exacerbation syndrome.
There is a lot of research activity on biomarkers of bacterial infection in CES. The systematic review conducted by the authors of these guidelines, focusing on traditional exacerbation of COPD and excluding pneumonia, suggests that both C-reactive protein (CRP) and procalcitonin (PCT) are useful in guiding antibiotic treatment, as they are associated with lower prescription of these types of drugs, with no differences in the rates of treatment failure, recurrence, hospitalization or mortality (Table 5, Appendix B, Annex 1). CRP can be evaluated in both hospital and outpatient settings by determination in capillary blood.29,30 In patients with elevated CRP (≥ 20 mg/L), even with inconclusive sputum, the use of antibiotics is suggested in patients with CES (Table 5, Appendix B, Annex 1). PCT is also useful in reducing antibiotic use.31,32 However, it is not recommended for routine use in COPD exacerbations due to its higher cost, lower availability, and the possibility of using clinical parameters, such as sputum color or more accessible biomarkers such as CRP (Table 5, Appendix B, Annex 1). The use of PCT in community-acquired pneumonia or nosocomial pneumonia is not recommended either.33,34
Summary of PICO questions.
| PICO question | Recommendation | Specifications | Strength of recommendation | Level of evidence |
|---|---|---|---|---|
| 1. Should antibiotics be prescribed for COPD exacerbations? | Antibiotics are suggested in patients with an outpatient COPD exacerbation. | Many of the exacerbations treated with placebo do not present treatment failure, suggesting that the antibiotic is not always necessary. A change in sputum color (from mucoid to dark) is associated with greater isolation of potentially pathogenic microorganisms and is therefore considered a useful parameter for antibiotic administration. If the sputum is inconclusive, the use of CRP (≥20 mg/L) is also considered useful for the administration of antibiotics. Patients who require ventilatory support have a higher risk of bacterial infection. Antibiotics are also recommended in these circumstances. | Weak | Low |
| Antibiotics are suggested for patients with COPD exacerbation who require hospital admission. | Weak | Low | ||
| Antibiotics are recommended for all patients who have COPD exacerbation and require ICU admission. | Strong | Moderate | ||
| 2. Is a change in sputum color useful in guiding the administration of antibiotics in patients with COPD exacerbation? | Antibiotics are suggested when there is a change in sputum color (from mucoid to dark) in patients with COPD exacerbation. | Weak | Very low | |
| 3. Is the determination of C-reactive protein (CRP) useful in guiding the administration of antibiotics in patients with COPD exacerbation? | It is suggested that antibiotic treatment should be guided with CRP determination in patients with COPD exacerbation in whom assessment of the change in sputum color is inconclusive. | Antibiotic treatment should be indicated in patients with elevated CRP (≥20 mg/L). This biomarker can be used in both hospital and outpatient settings (capillary CRP). | Weak | Low |
| 4. Is the determination of procalcitonin (PCT) useful in guiding the administration of antibiotics in patients with COPD exacerbation? | Routine use of procalcitonin to guide the use of antibiotics during COPD exacerbation is not recommended. | Procalcitonin is useful in reducing antibiotic use. However, it is not recommended for routine use in COPD exacerbations due to its higher cost, lower availability and the possibility of using clinical parameters, such as sputum color, or more accessible biomarkers such as CRP. | Weak | Low |
| 5. Should oral corticosteroids be prescribed in COPD exacerbations? | The use of oral corticosteroids in patients with severe exacerbation is recommended. | The recommended dose of oral corticosteroids is 0.5 mg/kg/day prednisone or equivalent, for up to 5 days in moderate exacerbations and up to 14 days in severe exacerbations. The parenteral route is preferable for very severe exacerbations. | Strong | Moderate |
| The use of oral corticosteroids in patients with moderate exacerbation is recommended. | Weak | Moderate | ||
| 6. Is the determination of eosinophil count useful in guiding the administration of oral corticosteroids in patients with COPD exacerbation? | The administration of oral corticosteroids is recommended in patients with eosinophil counts ≥ 300 cells/mm3. | The efficacy of oral corticosteroids in COPD exacerbations is higher in patients with eosinophil counts ≥ 300 cells/mm3. | Weak | Low |
COPD: chronic obstructive pulmonary disease; ICU: Intensive care unit; PICO: patient, intervention, comparison, and outcomes.
TTs are clinical, physiological, or biological characteristics present in each individual patient, which can be identified by diagnostic tests or biomarkers and have a specific treatment.4,35,36Table 6 lists the main TTs in CES.
Treatable traits of COPD exacerbation syndrome.
| Treatable traits | Biomarker | Treatment |
|---|---|---|
| Endotype-based TTs | ||
| Bacterial infection | Sputum color | Antibiotics |
| CRP (≥20 mg/L) | ||
| T2 inflammation | Peripheral eosinophilia (≥ 300 cells/mm3) | Systemic corticosteroids |
| Ventricular dysfunction | NT-proBNP | Diuretics, beta-blockers, |
| ARA-II, ACE inhibitors | ||
| Cardiac ischemia | Troponin | Antiplatelet agents, beta-blockers |
| Functional TTs | ||
| Acute hypoxemic respiratory failure | PaO2 less than 60 mmHg | Oxygen therapy |
| Acute hypercapnic respiratory failure | PaCO2 > 45 mmHg | Avoid sedatives |
| Respiratory acidosis | pH < 7.35 | Consider non-invasive ventilation. |
| Imaging TT (Chest X-ray/chest CT) | ||
| Pneumonia | Pulmonary parenchyma infiltrate | Antibiotics |
| Pulmonary embolism | Vascular filling defect | Anticoagulation |
| Pulmonary hypertension | Pulmonary artery/aorta ratio >1 | Consider oxygen therapy |
| Infectious bronchiolitis | Tree-in-bud sign | Consider antibiotics |
| Bronchiectasis | Bronchiectasis | Consider antibiotics |
| Lifestyle/behavioral TT | ||
| Low therapeutic adherence | Adherence questionnaires (TAI, etc.) | Health education |
| Incorrect inhalation technique | Review of the technique (questionnaires) | Training |
| Social problems | Social and dependency assessment (Pfeiffer, etc.) | Social support programs |
ACE inhibitors: angiotensin-converting enzyme inhibitor; ARA-II: Angiotensin II receptor antagonists; COPD: chronic obstructive pulmonary disease; CRP: C-reactive protein; NT-proBNP: N-terminal pro-brain or pro B-type natriuretic peptide; TT: treatable trait; Rx: radiography; CT: computed tomography.
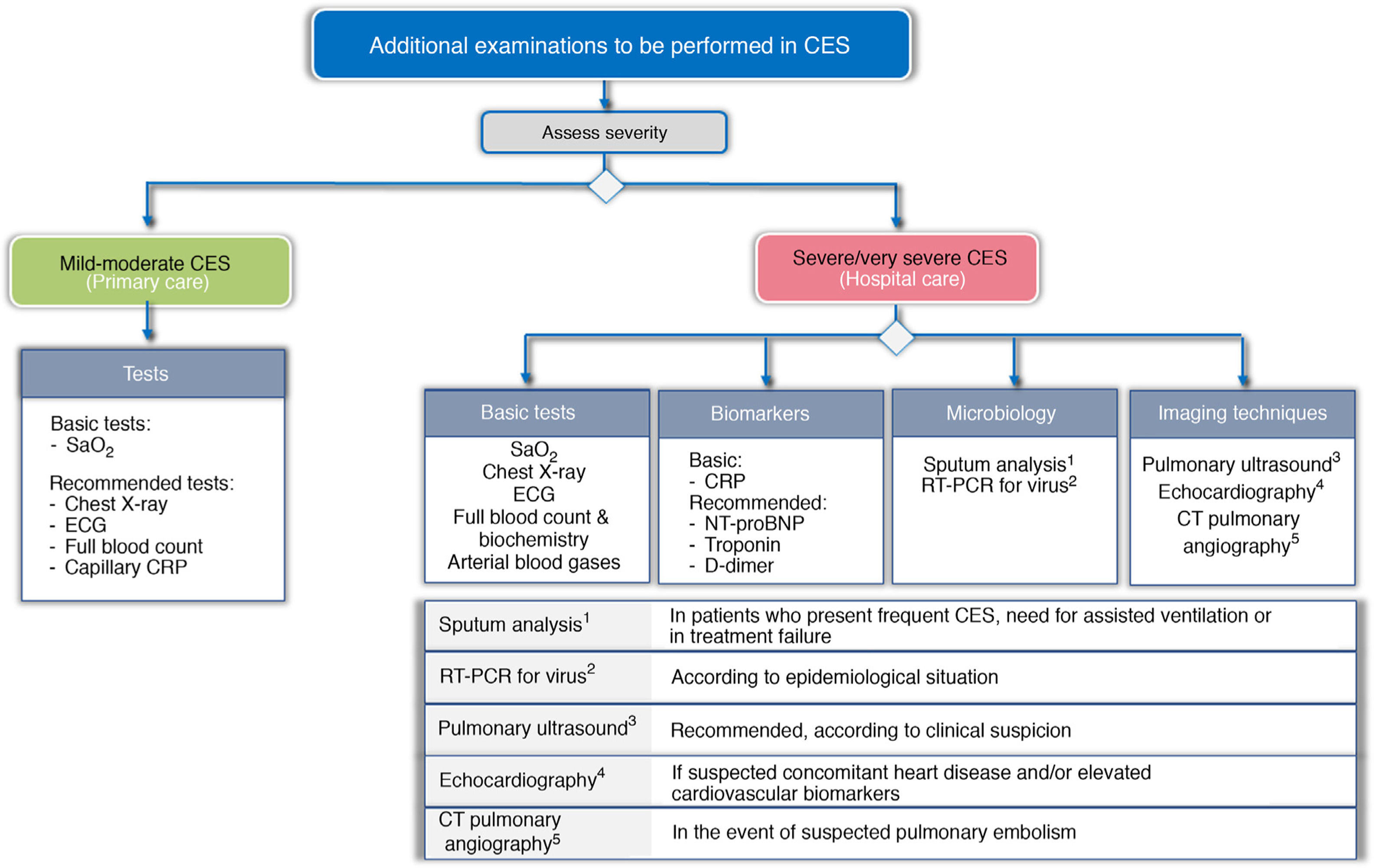
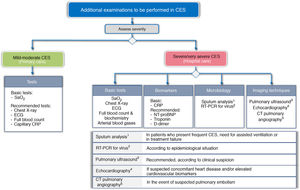
To try to establish the severity, and to identify the trigger and TTs, a different approach is proposed according to the level of care where the CES is treated. Fig. 3 shows the basic and recommended additional examinations, depending on the level of care.
Recommended additional examinations for CES, depending on level of care.
CRP: C-reactive protein; CTPA: computed tomography pulmonary angiography; ECG: electrocardiogram; NT-proBNP: N-terminal pro-brain natriuretic peptide; RT-PCR: reverse transcriptase polymerase chain reaction; SaO2: Arterial oxygen saturation
In the primary care setting, the determination of arterial oxygen saturation (SaO2) is considered basic, and an X-ray, electrocardiogram (ECG) and laboratory tests are recommended, together with capillary determination of CRP.
In the hospital, the study should be much more comprehensive. GesEPOC considers chest X-ray, ECG, arterial blood gases and laboratory tests (blood count and routine biochemistry), including at least the determination of CRP, as basic tests to be performed. The use of other biomarkers such as troponin, N-terminal pro-brain natriuretic peptide (NT-proBNP), or D dimer is also recommended. Screening for viruses using RT-PCR panels should be considered according to the epidemiological situation. Echocardiography is indicated for suspected concomitant heart disease or elevated cardiovascular biomarkers. CT pulmonary angiography (CTPA) should be considered for suspected pulmonary embolism, and the use of pretest clinical probability tools such as the Wells or Geneva score37 is recommended.
Lung ultrasound has been developed in recent years and has been shown to be useful for the rapid bedside diagnosis of decompensated heart failure, pneumonia, pulmonary thromboembolism, pneumothorax, pleural and pericardial effusion, and diaphragmatic dysfunction.38•42 There are different systematic approaches, such as the Bedside Lung Ultrasonography in Emergency (BLUE) protocol, with an overall diagnostic accuracy ranging from 77.5% to 90.5% in patients with acute dyspnea.41,42 The sensitivity and specificity for classical exacerbation of COPD is somewhat lower (sensitivity 98.2% and specificity 69%).42 Nevertheless, these types of comprehensive approaches based on lung ultrasound can be very useful for the new syndromic approach to CES.
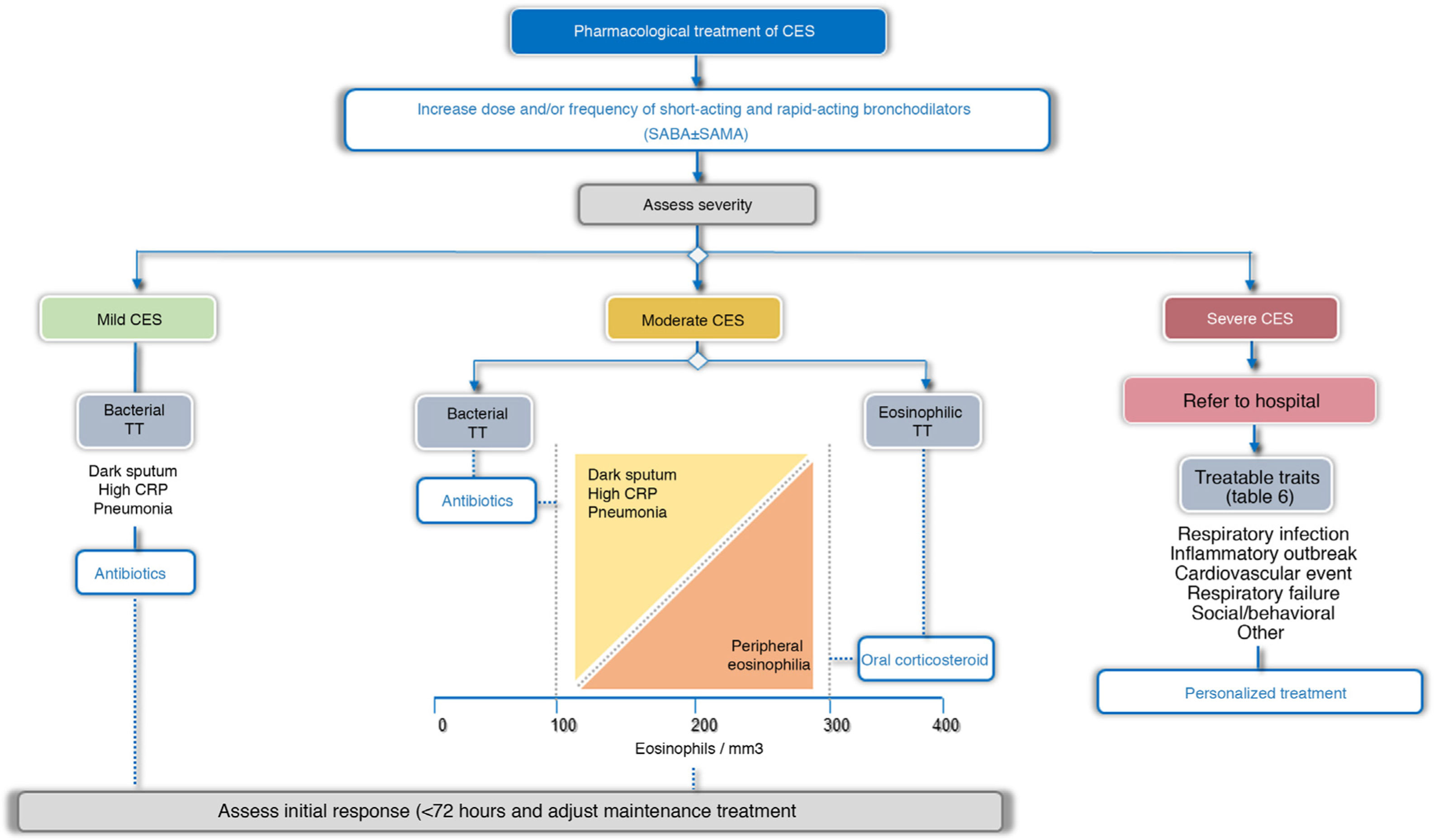
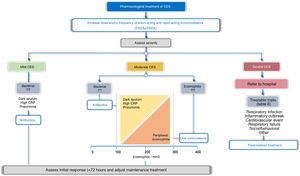
TreatmentTreatment guidelines should be tailored to each patient based on possible triggers, severity, and TTs identified. In this respect, 2 treatment scenarios are identified: outpatients and inpatients (Fig. 4). More than 80% of exacerbations are managed on an outpatient basis.43Table 5 summarizes the main PICO questions for the treatment of CES.
Bronchodilator treatment for immediate relief of symptoms is considered essential for all patients, while the use of antibiotics, systemic corticosteroids, oxygen therapy, assisted ventilation, or treatment of the comorbidity itself will vary depending on the severity or potential TT of the CES (Fig. 4, Table 6).
Pharmacological treatmentBronchodilatorsIn CES of any intensity, the main intervention is the optimization of bronchodilation, increasing the dose or frequency of bronchodilators.44 Short-acting bronchodilators are the treatment of choice for CES. Rapid-acting drugs such as β2-agonists (salbutamol and terbutaline) should be used, and a short-acting anticholinergic (ipratropium) may be added if necessary.
Pressurized metered-dose inhalers (pMDI) or nebulizers can be used to deliver inhaled drugs during the exacerbation. A systematic review of the forms of administration of short-acting bronchodilators concluded that when the inhalation technique is good, there are no significant differences in forced expiratory volume in the first second (FEV1) between MDIs with or without a spacer device and nebulizers.45
The recommended doses are: salbutamol, 400 to 600 µg/ 4 to 6 h (4-6 inhalations/4-6 h) or terbutaline 500 to 1000 µg/4-6 h (1-2 inhalations/6 h); and for ipratropium, 80 to 120 µg/4-6 h (4-6 inhalations c/4-6 h). In the case of nebulized medication, the dosage should be 2.5 to 5 mg salbutamol and/or 0.5 to 1 mg ipratropium every 4 to 6 hours.
Long-acting bronchodilators are the background treatment for COPD but their efficacy in CES is not sufficiently documented.46 However, it is important to remember that if the patient is already using a long-acting bronchodilator to control their underlying disease, these should not be discontinued during outpatient CES treatment.
AntibioticsIn exacerbations in outpatients and in inpatients, the use of antibiotics reduces the risk of treatment failure and increases the time until the next exacerbation, without affecting health-related quality of life (HRQoL), recurrences or mortality.47•49 In cases requiring admission to the intensive care unit (ICU), antibiotic treatment has been associated with a significant reduction in all-cause mortality, reduced treatment failure, and shorter length of hospital stay.47,50 In general, antibiotics are recommended for use during an outpatient or inpatient CES, and are particularly recommended for administration in all patients requiring ICU admission (Table 5, Appendix B, supplement 1). Many CES treated with placebo do well, suggesting that antibiotics are not always necessary. Antibiotic administration should be particularly indicated when there is a change in sputum color (from mucoid to dark),47•49 when the patient requires ventilatory assistance, both invasive and non-invasive,50 and in cases with elevated CRP (≥ 20 mg/dL), even if the appearance of the sputum is inconclusive.29,30 Antibiotics are also recommended for all patients with CES who present with pneumonia, in accordance with the recommendations set out in clinical practice guidelines for the management of pneumonia.33,34,51
Table 7 shows the main antibiotics to consider during CES. This choice will depend on determining the bacterial species involved, local antibiotic resistance, the severity of the exacerbation itself and the risk of Pseudomonas aeruginosa infection. This risk is defined by the use of 4 or more antibiotic treatment cycles in the previous year, lung function with FEV1 < 50% of predicted, the presence of significant bronchiectasis or the prior isolation of Pseudomonas in sputum in stable COPD or in a previous exacerbation.1,52,53
Recommendations on the use of antibiotics in CES.
| Severity of exacerbation | Microorganisms | Antibiotic of choice |
|---|---|---|
| Mild CES | H. influenzae | Amoxicillin-clavulanic acid |
| S. pneumoniae | Cefditorene | |
| M. catarrhalis | Levofloxacina | |
| Moxifloxacina | ||
| Moderate CES | Same as group A + S. pneumoniae with reduced sensitivity to penicillin. | Amoxicillin-clavulanic acid |
| Enterobacteria | Cefditorene | |
| Levofloxacina | ||
| Moxifloxacina | ||
| Severe-very severe CES with no risk of Pseudomonas infection | Same as group A + S. pneumoniae with reduced sensitivity to penicillin. | Amoxicillin-clavulanic acid |
| Enterobacteria | Ceftriaxone | |
| Cefotaxime | ||
| Levofloxacina | ||
| Moxifloxacina | ||
| Severe-very severe CES with risk of Pseudomonas infection | Same as group B + P. aeruginosa | β-lactam with anti-pseudomonal activityb |
| Alternative: quinolonesa with antipseudomonal activityc | ||
CES: chronic obstructive pulmonary disease exacerbation syndrome.
Systemic corticosteroids have been shown to accelerate the recovery of symptoms, improve lung function and reduce treatment failures, albeit without reducing mortality.47,54 However, recent studies suggest that these drugs may be less effective in patients with low blood eosinophil counts.55,56 According to the systematic review carried out by the authors of these guidelines, the use of oral corticosteroids in patients with severe or very severe CES is recommended and their use for moderate CES is suggested (Table 5, Annex 1). The efficacy of these drugs in CES is higher in patients with eosinophil counts ≥ 300 cells/mm3. The recommended dose is 0.5 mg/kg/day prednisone or equivalent for up to 5 days in moderate CES and up to 14 days in severe or very severe CES.47,57 Although no differences have been observed between intravenous or oral administration,47 the parenteral route is preferable for very severe CES.
Prophylaxis of venous thromboembolismSevere or very severe CES carry an elevated risk of venous thromboembolism (VTE), so low molecular weight heparins are recommended for use at prophylactic doses in high-risk patients.58 Their use is also indicated in moderate CES where the patient remains in bed or is inactive for ≥ 3 days.
Optimize treatment of the comorbidityIn COPD, the co-existence of different comorbidities, such as high blood pressure, ischemic heart disease, arrhythmias, heart failure, and diabetes, is common. It will therefore be necessary to optimize their treatment, in accordance with the recommendations of the specific clinical practice guidelines. In patients with heart disease, both β2-agonists and short-acting anticholinergics have been associated with an increase in arrhythmias, so caution should be exercised with the dose used, especially with nebulized devices.59
Non-pharmacological treatmentOxygen therapyThe administration of supplemental oxygen is one of the cornerstones in the treatment of severe CES in COPD with respiratory failure. The goal of oxygen therapy is to achieve an SaO2 between 88% and 92%. However, oxygen should be administered in a controlled manner, as in some patients the principal stimulus of the respiratory center depends on the degree of hypoxemia rather than on the usual hypercapnic stimulus. Uncontrolled oxygen administration can lead to suppression of the respiratory stimulus, carbon dioxide narcosis, and even respiratory arrest. In clinical practice, low inspiratory oxygen concentrations, either 24% or 28%, should be administered via high-flow venturi masks or nasal prongs at low flow rates of 2 to 4 L/min.
High-flow oxygen therapyHigh-flow oxygen (HFO) therapy allows gas to be delivered at high flow rates (up to 60 L/min) with variable air and oxygen ratios via a nasal cannula, with gas delivered at ideal temperature and humidity levels (37°C and 100% relative humidity). In CES, HFO has been shown to improve oxygenation and ventilation, decreasing hypercapnia and improving HRQoL.60•62 Some non-controlled trials and a recent randomized non-inferiority trial have compared the use of HFO versus non-invasive ventilation (NIV) in COPD patients with mild-moderate acidosis (pH: 7.25 to 7.35). They found no significant differences, although tolerance was better for the HFO.63•65 Despite these potential benefits, more evidence is needed to establish specific recommendations for use in CES.
Assisted ventilationIn cases of severe ventilatory failure, with altered level of consciousness or respiratory acidosis, despite optimal medical treatment, the use of ventilatory support should be considered.1 Mechanical ventilation can be administered non-invasively or invasively (IV).
Non-invasive mechanical ventilationIn patients with acute hypercapnic respiratory failure, NIV reduces mortality, the need for intubation, and treatment complications compared with usual therapy without ventilatory support. Hospital and ICU stays are also reduced.1,47,66Table 8 shows the indications and contraindications for NIV.
Indications and relative contraindications for non-invasive ventilation (NIV).
| Indications |
| Respiratory acidosis (pH < 7.35) with hypercapnia (PaCO2 >45 mmHg) despite optimal treatment |
| Contraindications |
| Respiratory arrest |
| Cardiovascular instability |
| Drowsiness that prevents the patient from collaborating |
| High risk of aspiration |
| Recent facial or gastroesophageal surgery |
| Nasopharyngeal abnormalities |
| Burns |
Invasive mechanical ventilation should be considered in cases of respiratory arrest, intolerance to or failure of NIV, worsening of respiratory acidosis (pH < 7.25), hemodynamic instability or decreased level of consciousness that does not improve with correct treatment.1
Early pulmonary rehabilitationIn patients hospitalized for COPD exacerbation, early pulmonary rehabilitation programs reduce readmissions, and improve HRQoL and exercise capacity.67 However, these results are variable depending on when the program is started. The decline in readmissions is only significant for programs initiated within 4 weeks after hospital discharge.
Hospital admission and discharge criteriaTable 9 shows the hospital admission criteria. The length of hospital stay varies from patient to patient, and there are insufficient data to establish the optimal length of hospitalization in individual patients with CES. At discharge, the background treatment should be adjusted, with special emphasis on reducing possible relapses and recurrences (Table 10).
Criteria for admission to the hospital ward.
| No improvement after correct treatment and 6-12 hours observation |
| Respiratory acidosis (pH < 7.35) |
| PaO2 < 55 mmHg |
| PaCO2 > 50 mmHg in patients with no previous hypercapnia |
| Need for non-invasive mechanical ventilation |
| Pneumonia, provided that the specific criteria for pneumonia severity that indicate admission are met |
| Presence of serious complications or comorbidities: |
| Pleural effusion |
| Pneumothorax |
| Venous thromboembolism |
| Chest trauma with fractured ribs |
| Cardiovascular disorders (heart failure, ischemic heart disease, uncontrolled arrhythmias) |
| Severe anemia |
| Insufficient home support |
Treatment recommendations at discharge.
| Abstain from smoking |
| Recommend regular exercise. Assess pulmonary rehabilitation after hospitalization. |
| Maintain and adjust the usual treatment, according to clinical phenotype and treatable traits: |
| Review the patient's inhalation technique |
| Non-exacerbator phenotype: LAMA + LABA |
| Non-eosinophilic exacerbator phenotype: LAMA + LABA. Adding inhaled corticosteroids (ICS) (triple therapy) may be considered in patients with peripheral blood eosinophil count >100 cells/mm3. |
| Eosinophilic exacerbator phenotype: LABA/ICS or triple therapy (LAMA + LABA + ICS), depending on the severity of symptoms. |
| Evaluate and treat different treatable traits. |
| Oxygen therapy: readjust as needed |
| Antibiotics if indications are met |
| Oral corticosteroids: 0.5 mg/kg/day for 5-14 days. |
| Home non-invasive mechanical ventilation. Consider NIV in patients with recurrent acidotic exacerbations and/or in patients who combine hypoventilation due to other causes (apnea-hypoapnea syndrome, obesity-hypoventilation, etc.) |
| Clinical follow-up in 72 hours and review in 2-4 weeks |
| Ensure treatment adherence |
| Nursing care plan |
| Ensure continuity of care |
ICS: inhaled corticosteroids; LABA: long-acting β2-adrenergic; LAMA: long-acting muscarinic antagonist.
For outpatient CES, follow-up monitoring is established 72 hours after treatment initiation, in the primary care setting, with the aim of assessing the course of the process and identifying the existence of possible early treatment failures. If the patient has required hospital admission, the initial follow-up monitoring should be carried out between 2 and 4 weeks after hospital discharge, and an additional visit at 8-12 weeks is recommended.
At the visit, the degree of control68•71 and the treatment response should be evaluated, cases with difficulty in understanding indications and special risk situations should be identified, and diagnostic, treatment adherence and tolerance aspects as well as the inhalation technique should be reviewed; emphasis should be placed on educational and preventive aspects and the involvement of patients and caregivers in self-care aspects should be encouraged. People with frequent hospitalizations may benefit from specific care programs to reduce admissions and improve HRQoL.72 For all patients without previous spirometry, confirmation spirometry should be performed in a stable phase.
FundingThis project has not received any type of funding.
Conflict of interestsJuan José Soler-Cataluña has received honoraria for scientific consultancy and/or for lecturing from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Grupo Ferrer, GlaxoSmithKline, Laboratorios Esteve, Teva, Menarini, Mundipharma, Novartis, Rovi and Zambon. Pascual Piñera declares that he has no conflict of interest. Juan Antonio Trigueros has received honoraria for training activities and participation in clinical trials from AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, GlaxoSmithKline, Mundipharma, Menarini, Pfizer and Teva. Myriam Calle has received honoraria for lecturing from Novartis, Chiesi, AstraZeneca, Boehringer Ingelheim and GlaxoSmithKline. Juan Ciro Casanova has received honoraria in the last 3 years for lecturing and/or scientific consultancy and/or grants for research projects from AstraZeneca, Bial, Boehringer-Ingelheim, Chiesi, GlaxoSmithKline, Menarini and Novartis. Borja G. Cosio has received honoraria for scientific consultancy and/or for lecturing from Chiesi, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Laboratorios Esteve, Faes Farma, Teva, Menarini, Sanofi and Novartis. Jose Luis Lopez-Campos has received honoraria in the last 3 years for giving lectures, scientific consultancy, participation in clinical studies or writing publications for: AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, Esteve, Ferrer, Gebro, GlaxoSmithKline, Grifols, Menarini, Novartis, Rovi and Teva. Jesús Molina has received honoraria in the last 3 years for scientific consultancy and/or for lecturing from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini, Novartis and Pfizer. Pere Almagro has received honoraria for scientific consultancy and/or for lecturing from Chiesi, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Laboratorios Esteve, Menarini and Novartis. José-Tomás Gómez has received honoraria for scientific consultancy and/or for lecturing from AstraZeneca, BIAL, Chiesi, Laboratorios Esteve, Grifols, GlaxoSmithKline, Mylan, Reig-Jofré, ROVI, TEVA and Zambon. Juan Antonio Riesco has received honoraria for scientific consultancy and/or for lecturing from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini, Mundipharma, Novartis, Pfizer, Rovi and Teva. Pere Simonet has received honoraria for continuing education activities from Boehringer Ingelheim, Menarini, Mundipharma, GlaxoSmithKline, Chiesi and AstraZeneca. David Rigau has no conflicts of interest. Joan B. Soriano has received funding for medical research and grants from 2016 to 2021 from Chiesi, GSK, Linde and Novartis via the Hospital Universitario de La Princesa Research Institute; he has participated in training activities, conferences, advisory boards and/or consultancy during the period 2015-2019 sponsored by: Air Liquide, Almirall, AstraZeneca, Boehringer-Ingelheim, CHEST, Chiesi, ERS, IHME, GEBRO, Grifols, GSK, laminar, Linde, Lipopharma, Menarini, Mundipharma, Novartis, Pfizer, RiRL, Rovi, SEPAR, Takeda and Zambon; he has not received (directly or indirectly) funds from the tobacco industry or its affiliates. Julio Ancochea has received honoraria for scientific consultancy and/or for lecturing from Actelion, Air Liquide, Almirall, AstraZeneca, Boehringer Ingelheim, Carburos Médica, Chiesi, Faes Farma, Ferrer, GlaxoSmithKline, InterMune, Linde Healthcare, Menarini, MSD, Mundipharma, Novartis, Pfizer, Roche, Rovi, Sandoz, Takeda and Teva. Marc Miravitlles has received speaker honoraria from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, AstraZeneca, Menarini, Rovi, Bial, Sandoz, Zambon, CSL Behring, Grifols and Novartis; consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Ferrer, GlaxoSmithKline, Bial, Gebro Pharma, Kamada, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, TEVA, Spin Therapeutics, pH Pharma, Novartis, Sanofi and Grifols, and research grants from Grifols.
Coordinator: Marc Miravitlles, Spanish Society of Pulmonology and Thoracic Surgery (SEPAR). Executive Committee: Pere Almagro, Spanish Society of Internal Medicine (SEMI); Julio Ancochea, Myriam Calle, Ciro Casanova, Eusebi Chiner, Borja G. Cosío, Elena Gimeno-Santos, Carme Hernández, José Luis López-Campos, Juan Antonio Riesco, Nuria Seijas, Joan B. Soriano, Juan José Soler-Cataluña (SEPAR); Jesús Molina, Spanish Society of Family and Community Medicine (semFYC); Dolors Navarro; Association of COPD and Sleep Apnea Patients and Families (APEAS), National Federation of Respiratory Patient Associations (FENAER), Spanish Patient Forum (FEP); Leopoldo Palacios Gómez, Federation of Community Nursing and Primary Care Associations (FAECAP); Pascual Piñera Salmerón, Spanish Society of Emergency Medicine and Emergencies (SEMES); Eulogio Pleguezuelos, Spanish Society of Rehabilitation and Physical Medicine (SERMEF), Society of Cardiorespiratory Rehabilitation (SORECAR); Sebastià Santaeugenia, Spanish Society of Geriatrics and Gerontology (SEGG); Pere Simonet, Respiratory Group in Primary Care (GRAP); José Tomás Gómez, Spanish Society of Primary Care Physicians (SEMERGEN); Juan Antonio Trigueros, Spanish Society of General and Family Physicians (SEMG). Methodology: David Rigau, Centro Cochrane Iberoamericano, Barcelona, Spain.