The Spanish COPD Guidelines (GesEPOC) were first published in 2012, and since then have undergone a series of updates incorporating new evidence on the diagnosis and treatment of COPD. GesEPOC was drawn up in partnership with scientific societies involved in the treatment of COPD and the Spanish Patients' Forum. Their recommendations are based on an evaluation of the evidence using GRADE methodology, and a narrative description of the evidence in areas in which GRADE cannot be applied. In this article, we summarize the recommendations on the pharmacological treatment of stable COPD based on 9 PICO questions. COPD treatment is a 4-step process: 1) diagnosis; 2) determination of the risk level; 3) initial and subsequent inhaled therapy; and 4) identification and management of treatable traits. For the selection of inhaled therapy, high-risk patients are divided into 3 phenotypes: non-exacerbator, eosinophilic exacerbator, and non-eosinophilic exacerbator. Some treatable traits are general and should be investigated in all patients, such as smoking or inhalation technique, while others affect severe patients in particular, such as chronic hypoxemia and chronic bronchial infection. COPD treatment is based on long-acting bronchodilators with single agents or in combination, depending on the patient's risk level. Eosinophilic exacerbators must receive inhaled corticosteroids, while non-eosinophilic exacerbators require a detailed evaluation to choose the best therapeutic option. The new GesEPOC also includes recommendations on the withdrawal of inhaled corticosteroids and on indications for alpha-1 antitrypsin treatment. GesEPOC offers a more individualized approach to COPD treatment tailored according to the clinical characteristics of patients and their level of complexity.
La Guía Española de la EPOC (GesEPOC) se publicó por primera vez en 2012 y desde entonces ha experimentado una serie de actualizaciones que incorporan las nuevas evidencias sobre el diagnóstico y tratamiento de la EPOC. GesEPOC es una guía de práctica clínica elaborada con la colaboración de las sociedades científicas implicadas en el tratamiento de la EPOC y del Foro Español de Pacientes. Sus recomendaciones se basan en una evaluación de la evidencia mediante la metodología GRADE y en una descripción narrativa de la evidencia en aquellas cuestiones en que la aplicación de GRADE no es posible. En este artículo se resumen las recomendaciones sobre el tratamiento farmacológico de la EPOC estable basadas en la elaboración de 9 preguntas PICO. El proceso de tratamiento de la EPOC comprende cuatro etapas: 1) diagnóstico; 2) determinación del nivel de riesgo; 3) tratamiento inhalado inicial y de continuación y 4) identificación y abordaje de los rasgos tratables. Para la elección del tratamiento inhalado los pacientes de alto riesgo se dividirán en tres fenotipos: no agudizador, agudizador eosinofílico y agudizador no eosinofílico. Los rasgos tratables comprenden unos de tipo general, que deben investigarse en todos los pacientes, como el tabaquismo o la técnica inhalatoria y otros más específicos, que afectan sobre todo a los pacientes graves, como la hipoxemia crónica o la infección bronquial crónica. La base del tratamiento de la EPOC la constituyen los broncodilatadores de larga duración en monoterapia o en combinación según el nivel de riesgo del paciente. Los pacientes agudizadores eosinofílicos deben recibir corticosteroides inhalados y los no eosinofílicos requieren una evaluación detallada para elegir la mejor opción terapéutica. La nueva GesEPOC también incluye recomendaciones sobre la retirada de corticosteroides inhalados y sobre la indicación de tratamiento con alfa-1 antitripsina. GesEPOC supone una aproximación al tratamiento de la EPOC más individualizada según las características clínicas de los pacientes y su nivel de riesgo o de complejidad.
The Spanish chronic obstructive pulmonary disease guidelines (GesEPOC) 2021 are the fourth GesEPOC update to appear since the first version was published in 2012.1 Representatives from the scientific societies involved in the care of patients with chronic obstructive pulmonary disease (COPD) and the Spanish Patient Forum have participated in the development of these guidelines.
GesEPOC were the first clinical guidelines on COPD to propose treatment guided by clinical phenotypes and were widely implemented. An audit of respiratory outpatients in Spain (EPOCONSUL study) between May 2014 and May 2015 revealed that 46.3% of the medical records of patients with COPD already included phenotype classification according to GesEPOC 2012.2
Diagnostic and treatment guidelines must be periodically updated to keep abreast of ongoing research and new evidence in COPD. This article presents the section on pharmacological treatment of stable COPD of the new GesEPOC 2021. The guidelines have been developed following GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodology.3 In this edition of the guidelines, 5 new PICO questions have been formulated and the recommendations of 4 questions from the GesEPOC 2017 edition4 have been retained, since no significant new evidence has emerged on these aspects of treatment. Details of the protocol, including the PICO questions (Patient, Intervention, Comparison, and Outcomes), literature search and evidence tables can be found in Supplement 1.
Care of the patient with COPDGesEPOC 2021 proposes a 4-step patient assessment process: 1) diagnosis of COPD and general measures; 2) risk stratification; 3) selection of inhaled treatment according to symptoms and clinical phenotype; and 4) identification and management of treatable traits.
DiagnosisThe process starts with diagnostic suspicion in an adult smoker or former smoker of more than 10 pack-years or chronic exposure to noxious partciles or gases who presents respiratory symptoms (dyspnea or chronic cough with or without associated expectoration). Diagnosis is confirmed by spirometry performed during clinical stability that demonstrates a post-bronchodilator ratio between the forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) of less than 0.7. However, it should be noted that this value can underestimate obstruction in young subjects and overdiagnose older subjects, as the ratio decreases physiologically with age.5,6 Therefore, 3 criteria must be met to establish a diagnosis of COPD: prior exposure to risk factors; respiratory symptoms; and obstruction in post-bronchodilation spirometry.
After diagnosis, a number of general measures should be addressed in all patients with COPD, including smoking cessation, proper nutrition, regular physical activity adapted to the patient's age and physical conditions, and evaluation and treatment of comorbidities. Not all of these general measures will be discussed in this publication. Alpha-1 antitrypsin (AAT) should be determined in all patients, and the specific management of AAT deficiency is described in the treatable traits section of these guidelines.
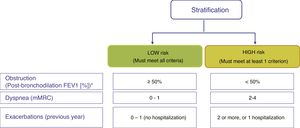
Risk stratificationThe risk level must then be evaluated. This is defined as the likelihood that the patient will present exacerbations, disease progression, future complications, higher use of healthcare resources or higher mortality. GesEPOC proposes classification into 2 risk levels: low and high. This risk classification does not imply referral between levels of care.
Factors considered for risk assessment are the degree of obstruction measured by post-bronchodilator FEV1 (%), grade of dyspnea measured by the modified Medical Research Council (mMRC) scale, and the history of exacerbations during the previous year (Fig. 1). The components of this risk classification have been shown to be predictive of mortality.7 The inclusion of FEV1 has been shown to significantly add predictive value to the risk classification,8 and recent studies have demonstrated that the risk classification is adaptable to real-world care situations and contributes to the selection of pharmacological treatment.9 The higher the risk level, the greater the need for therapeutic interventions (Table 1).
Adaptation of the care level to risk levels.
| Therapeutic interventions | ||
|---|---|---|
| Low risk | Smoking cessation | Counseling Specific treatment |
| Therapeutic education | Structured therapeutic education program aimed at:• Promoting self-care• Therapeutic adherenceInhalation technique | |
| Physical activity | Regular exercise | |
| Vaccination | Anti-influenzaAnti-pneumococcal (13-valent conjugate)Covid-19Assess dTpa | |
| Alpha-1 antitrypsin deficiency | Augmentation treatment according to guidelines | |
| Pharmacological treatment | Bronchodilators | |
| Comorbidity | Treatment of the comorbidity | |
| High risk | Add to previous treatment: | |
| Pharmacological treatment | Guided by clinical phenotypeIdentify treatable traits | |
| Non-pharmacological treatment | Pulmonary rehabilitationAssess long-term home oxygen therapyAssess non-invasive ventilationAssess lung volume reduction in patients with extensive emphysemaAssess lung transplant | |
Covid-19: coronavirus disease 2019; dTpa: Diphtheria, tetanus, acellular pertussis.
The general treatment objectives for COPD are to alleviate disease symptoms, reduce the frequency and severity of exacerbations, improve quality of life (QoL), and extend survival. Both short-term benefits (disease control) and mid- to long-term objectives (risk reduction) must be achieved.
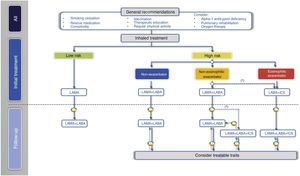
With regard to the initial pharmacological treatment, GesEPOC guidelines propose treatment based on the administration of inhaled drugs guided by symptoms in low-risk patients and by clinical phenotype in high-risk patients.10 The key points in the pharmacological treatment of COPD are presented in Table 2.
Key points in the pharmacological treatment of COPD.
|
|
|
|
|
|
|
COPD: chronic obstructive pulmonary disease; ICS: inhaled corticosteroids; LABD: long-acting bronchodilators.
No type of oral or anti-inflammatory treatment is indicated in the low-risk patient, and pharmacological treatment will consist of long-acting bronchodilators (LABD). In the rare case of mild obstruction with few or intermittent symptoms, short-acting bronchodilators (SABD) on demand may be indicated.
SABD can be of 2 types: anti-cholinergics (SAMA, short-acting muscarinic antagonists) such as ipratropium bromide, and short-acting beta-2 agonists (SABA) such as salbutamol or terbutaline. In patients with occasional symptoms, SABD treatment reduces symptoms and improves exercise tolerance.11 Because of their rapid onset of action, these drugs, added to the baseline treatment, are preferred for on-demand treatment of symptoms, regardless of the level of disease severity. In patients with persistent symptoms or limitations in their daily activities as a result of their respiratory problem, regular baseline treatment with a LABD will be required.
LABD can be beta-2 adrenergics (LABA, long-acting beta-agonists) or anticholinergics (LAMA, long-acting muscarinic antagonists). The LABA available in Spain are salmeterol, formoterol, indacaterol, olodaterol and vilanterol, and the LAMA are tiotropium, aclidinium, glycopyrronium and umeclidinium (Table 3). LABD should be used as a first step in the treatment of all patients with persistent symptoms who require regular treatment, because they enable better control of symptoms than can be achieved with SABD and improve lung function, exercise capacity and QoL.12,13 Furthermore, LABD – both LABA and LAMA – have been shown to reduce the number of exacerbations.14
Characteristics of the inhaled drugs for the treatment of COPD.
| Active substance | Presentation | Recommended dose | |
|---|---|---|---|
| Beta-2 adrenergics | Salbutamol | pMDI: 100 μg/inh | 200 μg/4–6 h |
| Terbutaline | Turbuhaler®: 500 μg/inh | 500 μg/6 h | |
| Salmeterol | pMDI: 25 μg/inh | 50 μg/12 h | |
| Accuhaler®: 50 μg/inh | |||
| Formoterol | pMDI: 12 μg/inh | 12 μg/12 h | |
| Turbuhaler®: 9 μg/inh | |||
| Aerolizer®: 12 μg/inh | |||
| Indacaterol | Breezhaler®: 150 μg/inh | 150 μg/24 h | |
| Breezhaler®: 300 μg/inh | |||
| Olodaterol | Respimat®: 2.5 μg/inh | 5 μg/24 h | |
| Anticholinergics | Ipratropium bromide | pMDI: 20 μg/inh | 20–40 μg/6–8 h |
| Tiotropium bromide | Handihaler®: 18 μg/inh | 18 μg/24 h | |
| 5 μg/24 h | |||
| Respimat®: 2.5 μg/inh | |||
| Aclidinium | Genuair®: 340 μg/inh | 340 μg/12 h | |
| Glycopyrronium | Breezhaler®: 50 μg/inh | 50 μg/24 h | |
| Umeclidinium | Ellipta®: 62.5 μg/inh | 62.5 μg/24 h | |
| LABA/LAMA | Indacaterol/glycopyrronium | Breezhaler®: 110/50 μg/inh | 110/50 μg/24 h |
| Aclidinium/formoterol | Genuair®: 340/12 μg/inh | 340/12 μg/12 h | |
| Umeclidinium/vilanterol | Ellipta®: 62.5/25 μg/inh | 62.5/25 μg/24 h | |
| Tiotropium/olodaterol | Respimat®: 2.5/2.5 μg/inh | 5/5 μg/24 h | |
| LABA/ICS | Beclomethasone/formoterol | Nexthaler®: 100/6 μg/inh | 200/12 μg/12 h |
| pMDI Modulite® 100/ μg/inh | |||
| Formoterol/budesonide | Turbuhaler®: 4.5/160 and 9/320 μg/inh | 9/320 μg/12 h | |
| Spiromax®: 4.5/160 and 9/320 μg/inh | |||
| Easyhaler®: 4.5/160 and 9/320 μg/inh | |||
| Salmeterol/fluticasone propionate | Accuhaler®: 50/500 μg/inh | 50/500 μg/12 h | |
| Forspiro®: 50/500 μg/inh | |||
| Fluticasone furoate/vilanterol | Ellipta®: 100/25 μg/inh | 100/25 μg/24 h | |
| LABA/LAMA/ICS | Beclomethasone/formoterol/glycopyrronium | pMDI: 100/6/12.5 μg/inh | 200/12/25 μg/12 h |
| Fluticasone furoate/vilanterol/umeclidinium | Ellipta®: 100/25/62.5 μg/inh | 100/25/62.5 μg/24 h |
LABA/ICS: long-acting beta-2 adrenergic/inhaled corticosteroid; LABA/LAMA: long-acting beta-2 adrenergic/long-acting muscarinic antagonist; pMDI: pressurized metered-dose inhaler; inh: inhalation.
There are differences between the different LABD; some have a duration of action of 12 h (aclidinium, salmeterol and formoterol) and others 24 h (tiotropium, umeclidinium, glycopyrronium, indacaterol, olodaterol and vilanterol). In terms of preventing exacerbations, tiotropium has been shown to be more effective than salmeterol or indacaterol15,16; for this reason, when choosing an LABD as monotherapy, a LAMA is recommended as first choice over a LABA (Table 4).
Recommendations on the pharmacological treatment of COPD in a stable phase.
| PICO question | Recommendation | Specifications | Strength of recommendation | Level of evidence |
|---|---|---|---|---|
| 1. What is the treatment of choice as monotherapy, a LABA or a LAMA?* | In patients with COPD who require a long-acting bronchodilator as monotherapy, treatment with a LAMA is suggested. | The evidence analyzed is based on greater prevention of exacerbations in studies conducted with tiotropium (a LAMA). In patients with no exacerbations, there are no differences in clinical efficacy between LAMA and LABA. | Weak | Moderate |
| 2. For initial treatment in a low-risk non-exacerbator patient, is dual bronchodilation superior to monotherapy? | In low-risk patients who remain symptomatic with a long-acting bronchodilator, dual bronchodilaton therapy is recommended. | In patients with severe or very severe spirometric impairment, therapy with dual bronchodilation is superior to monotherapy to begin with, due to its greater effect on lung function and symptoms. | Strong | Moderate |
| In high-risk symptomatic patients (mMRC >2), dual bronchodilation therapy is recommended over single bronchodilator therapy. | ||||
| 3. For initial treatment in a non-eosinophilic exacerbator patient, what is the treatment of choice, LABA/LAMA or LABA/ICS? | Initial treatment with LABA/LAMA is suggested in non-eosinophilic exacerbator patients. | The comparison between the efficacy of both alternatives in the prevention of exacerbations is inconclusive, although the benefit/risk ratio appears to favor LABA/LAMA treatment in the profile of patients requiring initial treatment. | Weak | Low |
| Treatment with LABA/ICS is an alternative in patients with very frequent exacerbations and blood eosinophilia close to 300 cells/mm3. | ||||
| 4. In patients with exacerbations despite treatment with LABA/LAMA, is triple therapy with LABA/LAMA/ICS effective? | In patients with exacerbations despite treatment with LABA/LAMA, triple therapy with LABA/LAMA/ICS is suggested. | Triple therapy with LABA/LAMA/ICS offers a greater reduction in the risk of exacerbations and a greater improvement in symptoms than dual bronchodilation (LABA/LAMA). | Weak | Moderate |
| It cannot be ruled out that the reduction in exacerbations with triple therapy versus LABA/LAMA in non-eosinophilic exacerbator patients is clinically irrelevant. | ||||
| 5. Is augmentation treatment with AAT effective in slowing the progress of emphysema in patients with AAT deficiency? | Augmentation treatment is suggested in patients with AAT deficiency emphysema with the aim of reducing the loss of lung density measured by CT. | Augmentation treatment, however, has not demonstrated efficacy in symptoms or reduction of exacerbations. | Weak | Moderate |
| 6. When should mucolytics be used to prevent exacerbations?* | In patients with the COPD exacerbator phenotype despite adequate treatment, it is suggested that a high-dose mucolytic be added. | The costs associated with treatment with mucolytic agents should be discussed with the patient. | Weak | Moderate |
| 7. When should roflumilast be used to prevent exacerbations?* | Roflumilast has been suggested as a second-line drug to prevent exacerbations in patients with the exacerbator phenotype with chronic bronchitis and severe airflow limitation. | Due to its safety profile, tolerance to the drug may be poor, and physicians should be aware of the possible onset of adverse effects. | Weak | Moderate |
| 8. When should long-term macrolides be used to prevent exacerbations?* | In patients with COPD with an exacerbator phenotype, with at least 3 exacerbations the previous year despite adequate treatment, long-term treatment with macrolides is suggested. | Treatment should be restricted to patients with severe COPD with frequent exacerbations. Possible adverse effects such as prolongation of the QT interval, hearing loss or development of resistance should be strictly monitored. | Weak | Moderate |
| 9. Can inhaled corticosteroids be withdrawn in patients with COPD? | a) Consider withdrawing ICS in patients with infrequent exacerbations (0–1 moderate in the previous year) and <300 eosinophils/mm3 | Treatment with long-acting bronchodilators should continue after withdrawal. | a) Weak | Moderate |
| b) Strong | Moderate | |||
| b) It is recommended not to withdraw ICS in eosinophilic exacerbator patients |
LABD are generally well tolerated and have few adverse effects. Nevertheless, the following should be considered: LABA has been associated with fine tremor of the extremities, muscle cramps, tachycardia, high blood pressure, peripheral vasodilatation, headache, hyperglycemia, hypokalemia, cough, bronchospasm, oropharyngeal irritation and dyspepsia, while LAMA may be associated with dry mouth, urinary retention, increased ocular pressure, and pharyngeal irritation. It should be noted that clinical trials exclude patients with significant heart disease, so clinicians should be vigilant with the use of bronchodilators in these patients.12,13
In symptomatic patients or those with clear exercise limitation despite bronchodilator monotherapy, treatment compliance and proper inhalation technique must be verified; there is also the possibility of switching LABD, for example, from a LAMA to a LABA.17 The next therapeutic step is dual bronchodilator therapy; the combination of LABA and LAMA offers an added functional benefit, with less need for rescue medication and improvement in symptoms and QoL compared to monotherapy.12,13,18,19
In the next treatment step in low-risk patients, two LABDs can be combined to optimize the bronchodilator effect (Fig. 2, Table 3).
Inhaled treatment in high-risk patientsIn high-risk patients, GesEPOC 2021 recognizes three phenotypes in the pharmacological treatment scheme: 1) non-exacerbator; 2) eosinophilic exacerbator; and 3) non-eosinophilic exacerbator.
This distribution of phenotypes differs from that of GesEPOC 2017,4 and is led by several pieces of evidence. First, the so-called mixed asthma-COPD phenotype (ACO, asthma-COPD overlap) was defined by GesEPOC and the Spanish Guidelines on the Management of Asthma (GEMA) as a patient with COPD and a concomitant diagnosis of asthma or with peripheral eosinophilia or a strongly positive bronchodilator test.20,21 Evidence has accumulated in recent years on the predictive role of peripheral eosinophilia in the clinical response to inhaled corticosteroids (ICS) in COPD. However, it has been found that a strongly positive bronchodilator test rarely occurs in isolation and has little diagnostic value.24,25 For this reason, GesEPOC proposes separating the two main clinical forms of ACO,26 reserving the name ACO for the coexistence of asthma as a comorbidity in a patient with COPD, requiring both diseases to be treated. In contrast, the presence of peripheral eosinophilia (>300 cells/μL) in a patient with a history of repeated exacerbations (2 or more in the last year or 1 with hospital admission) will define the eosinophilic exacerbator COPD patient.
In terms of the emphysema and chronic bronchitis phenotypes, both are well defined.1 However, there is no difference between them with regard to inhaled treatment of COPD. For this reason, we will include emphysema, as well as chronic cough and expectoration, in the list of treatable traits, as they will be useful in severe patients for the indication of other specific non-inhaled or non-pharmacological treatments. Thus, the GesEPOC 2021 phenotypes are defined as:
Non-exacerbator phenotypeThe non-exacerbator phenotype is characterized by a maximum of 1 exacerbation in the previous year without requiring inpatient care. Initial treatment in a patient with high-risk non-exacerbator COPD is dual bronchodilation (Table 4). This recommendation is based on evidence of superior bronchodilator efficacy compared to monotherapy, accompanied by a significant improvement in dyspnea and QoL and a reduction in the use of rescue medication18,27; this has even been demonstrated in patients not receiving concomitant ICS.28 Existing combinations of LABD (LABA/LAMA) are presented in Table 3.
Eosinophilic exacerbator phenotypeAn exacerbator phenotype is defined as any patient with COPD who presents 2 or more outpatient exacerbations, or 1 or more severe exacerbations requiring inpatient care in the previous year.29 To differentiate the new event from relapse or therapeutic failure, these exacerbations must be separated by at least 4 weeks from the resolution of the previous exacerbation, or 6 weeks from the onset of symptoms.29 Due to the different response to pharmacological treatments, it is important to differentiate patients with an eosinophilic or non-eosinophilic phenotype. Patients with >300 eosinophils/mm3 in a stable phase will be classified as eosinophilic exacerbators. The blood eosinophil count can vary,30,31 so it will be preferable to have different determinations from the same period in which the frequency of exacerbations is evaluated in order to make a more reliable therapeutic decision. Extensive studies in Spain show that approximately 15%–25% of patients with stable COPD have >300 eosinophils/mm3.31,32
Exacerbator patients with an elevated blood eosinophil count (>300 cells/mm3) experience a better clinical response to ICS and justify the use of ICS combined with a LABA as a first option to reduce the risk of exacerbations22,23,33 (Table 3). The response gradient to ICS depends on the blood eosinophil count, so while these compounds may also be useful in patients with values <300 cells/mm3, they will become less effective as peripheral eosinophilia decreases.22,34
The next therapeutic step in eosinophilic exacerbator patients is triple ICS/LABA/LAMA therapy (Table 3 and Fig. 2). Recent studies of fixed triple therapy have shown greater efficacy in improving lung function and respiratory symptoms and a greater reduction in the risk of exacerbations than the LABA/ICS combination.35–37 Triple therapy has also shown a greater reduction in the risk of exacerbations than the LABA/LAMA combination, especially in patients who had a higher blood eosinophil count.35,36,38 These studies included symptomatic patients with frequent severe exacerbations despite regular treatment for their COPD; triple therapy is therefore considered a continuation treatment and not an initial treatment for COPD.39
Non-eosinophilic exacerbator phenotypeThis is a patient who meets the criteria for the exacerbator phenotype, but at the same time has <300 eosinophils/mm3 in peripheral blood. ICS are less effective in these patients, although this does not exclude them from treatment, particularly if the eosinophil count is >100 cells/mm.3,34
In these patients, the LABA/LAMA combination has shown a modest improvement in the prevention of exacerbations compared to LAMA,40,41 but offers the added benefit of improving symptoms and QoL compared to monotherapy.12,13,18,19 The systematic review and meta-analysis of the results of LABA/LAMA versus LABA/ICS combinations in the prevention of exacerbations show large differences in effectiveness, mainly due to the different inclusion criteria in the clinical trials. Moreover, none of these studies compared LABA/LAMA with LABA/ICS as initial treatment35,36,42 (Supplement 1). The LABA/ICS combination generally provides better results when eosinophil counts are higher (closer to 300 eosinophils/mm3), when the frequency of exacerbations is higher, and when previous exacerbations responded well to systemic corticosteroids.36,43 The LABA/LAMA combination, on the other hand, offers better results than LABA/ICS when eosinophil counts are lower and exacerbations are less frequent or require treatment with antibiotics; it also has a greater bronchodilator effect and a lower risk of pneumonia.42,43 Since GesEPOC 2021 considers concomitant asthma as a comorbidity that must be treated, since patients with >300 eosinophils/mm3 are classified as eosinophilic phenotypes and, furthermore, since most exacerbator patients have a low frequency of exacerbations, first-line treatment with LABA/LAMA is recommended in most non-eosinophilic exacerbator patients (Table 4). The LABA/ICS combination may be indicated when the frequency of exacerbations is higher, the exacerbations respond to systemic corticosteroids, there is no history of pneumonia, and the eosinophil count is closer to 300 eosinophils/mm3.
The non-eosinophilic exacerbator patient who presents frequent or severe exacerbations despite treatment with LABA/LAMA requires specialized care, and the presence of treatable traits should be investigated,44,45 as indicated in the corresponding section. In relation to inhaled treatment, ICS in the form of triple therapy may be useful if the patient has eosinophil counts between 100 and 300 cells/mm.3,35,36,43 In this case, the indication for ICS should reconsider the factors associated with greater effectiveness and safety of ICS, such as: 1) more frequent or severe exacerbations, 2) previous exacerbations that respond to oral corticosteroids, 3) no current smokers, and 4) no history of pneumonia.46 In contrast, the efficacy of ICS in patients with eosinophil counts <100 cells/mm3 is very limited and their use is not recommended to avoid adverse effects35,36,43,47 (Table 4). When treatment was initiated with LABA/ICS, escalation will be to triple therapy.
Identification and management of treatable traits. Non-inhaled treatmentThe term “treatable trait” is used to refer to a characteristic (clinical, physiological or biological) that can be identified by diagnostic tests or biomarkers and that has a specific treatment.44,45 It is important to keep in mind that a given patient is likely to have several treatable traits, and that all of them should be considered. When defining treatable traits, it is essential to aim to improve clinical outcomes for individual patients while minimizing unnecessary side effects in those less likely to respond to a particular treatment.
GesEPOC identifies the most important and common treatable traits to be investigated in high-risk patients on the basis of their relevance and applicability, with the exception of AAT deficiency, which is a trait that must be investigated in all COPD patients48 (Table 5). Information on non-pharmacological treatments for treatable traits has been the subject of other national and international documents, so we shall refer to them only in summary form.
Some of the most relevant treatable traits of COPD, particularly important in high-risk patients.
| Treatable traits | Indicators | Relevance and therapeutic implications |
|---|---|---|
| Alpha-1 antitrypsin deficiency* | Alpha-1 antitrypsin levels in peripheral blood | Associated with an increased risk of COPD and accelerated disease progression. Augmentation therapy prevents the progression of emphysema.48 |
| Dyspnea | Dyspnea scales. Computed tomography | Theophylline may improve dyspnea.49 |
| Pulmonary rehabilitation is effective in controlling dyspnea.96 In selected patients, lung volume reduction techniques may improve severe dyspnea.59 | ||
| Chronic bronchitis | Cough and sputum for 3 consecutive months for 2 years. | The presence of chronic bronchitis is a predisposing factor for exacerbations in COPD. |
| In the exacerbator phenotype with chronic bronchitis, roflumilast is effective in preventing exacerbations.70–72 Mucolytics/antioxidants are also effective in reducing exacerbations.67–70 | ||
| Severe emphysema and pulmonary hyperinflation | Computed tomography, measurement of lung volumes and CO diffusion capacity | • Lung volume reduction surgery in selected patients has been shown to improve exercise tolerance, health status, and lung function.59 |
| Chronic bronchial infection | Presence of potentially pathogenic microorganisms in respiratory sample cultures | Associated with exacerbations of infectious etiology, with greater frequency and severity, and higher mortality and functional decline. Long-term antibiotic treatment added to the usual medication can reduce exacerbations and improve quality of life.70,78,79 Mucolytics/antioxidants are also effective in reducing exacerbations.67–70 |
| Bronchiectasis | Computed tomography | Worse prognosis and greater frequency and severity of exacerbations. Follow treatment according to bronchiectasis guidelines75 |
| Precapillary pulmonary hypertension | Echocardiogram, natriuretic peptide, catheterization | This is a factor for poor prognosis, and treatment improves symptoms and prevents associated complications. |
| Chronic respiratory failure | PaO2 <60 mmHg and/or PaCO2 >45 mmHg | Chronic respiratory failure is associated with decreased survival. Continuous home oxygen therapy has been shown to increase survival and reduce exacerbations and hospitalizations.63 |
| In patients with sustained hypercapnia and recurrent episodes of respiratory acidosis, non-invasive ventilation has been shown to be useful.64 | ||
| Cachexia | Body mass index (BMI ≤ 20 kg/m2) | Malnutrition is associated with increased risk of hospitalization, longer length of stay, and increased risk of readmission. Nutritional supplements, diet and physical activity are the treatment recommendations.65,97 |
Dyspnea should be regarded as a symptom, but for reasons of text organization, we have included the treatment of severe dyspnea in this section. Patients who continue to have significant dyspnea despite dual bronchodilation require an evaluation that includes the possibility of identifying treatable traits and ruling out comorbidities that may increase dyspnea, such as heart failure. In particular, the need for a pulmonary rehabilitation program and a chest computed tomography (CT) to evaluate the indication for lung volume reduction techniques should be assessed.
From a pharmacological point of view, the addition of theophyllines may be attempted. These are weak bronchodilators that have a positive effect on diaphragm strength, increase performance of the respiratory muscles, reduce air trapping and improve mucociliary clearance.49,50 Although the usual dose is 200–300 mg/12 h orally in sustained-release tablets, good results have been obtained with doses of 200 or 300 mg/day in 24 h extended-release preparations.50 Lower doses (100 mg/12 h) have shown beneficial effects on pre-bronchodilator FEV1,51 although they have not demonstrated an effect on the prevention of exacerbations.52,53
Theophylline toxicity is dose-dependent. When administered on a long-term basis, plasma concentrations should be checked, and clinicians must take into account the risk of interactions with other drugs such as allopurinol, ciprofloxacin, erythromycin, benzodiazepines and cimetidine, among others. In any case, their limited clinical efficacy and narrow therapeutic margin relegate them to third-line therapy, mainly in high-risk patients if dyspnea persists after dual bronchodilator therapy.49
Alpha-1 antitrypsin deficiencyAlpha-1 antitrypsin (AAT) deficiency is a congenital cause of emphysema in adulthood.48 Severe AAT deficiency affects 1/4500 Caucasian people and is responsible for approximately 1 in every 700 cases of COPD in southern Europe.54 For correct identification, all patients with COPD should have at least 1 measurement of their serum AAT levels or determination of their genotype using non-invasive techniques such as DNA analysis obtained by buccal swabs.55 After a patient with AAT deficiency is identified, a family study should be carried out to identify possible undiagnosed cases.48 Any cases identified should be reported to the Spanish Registry of Patients with Alpha-1 Antitrypsin Deficiency, part of the EARCO (European Alpha-1 Research Collaboration) European registry,56 and the patient should be referred to a reference center for complete diagnosis and assessment for possible augmentation therapy and a family study.57
Augmentation therapy with purified AAT is indicated in patients with pulmonary emphysema associated with severe AAT deficiency, due to its effect in slowing down the loss of lung density58 (Table 4). Early diagnosis is important to avoid risk exposures, especially smoking, and to initiate specific treatment as soon as possible to preserve lung tissue.48
Severe emphysema and hyperinflationThe presence of severe emphysema with hyperinflation leads to severe dyspnea and exercise intolerance. Its evaluation requires detailed analysis with chest CT, lung volumes, CO diffusing capacity test and 6-minute walk test. Depending on the severity and distribution of emphysema, the patient may be eligible for endoscopic or surgical lung volume reduction techniques.59
Pulmonary arterial hypertensionPulmonary arterial hypertension also usually presents with severe dyspnea or dyspnea disproportionate to the degree of bronchial obstruction, and exercise intolerance with severe early desaturation during the 6-minute walk test (6MWT). It requires assessment with arterial blood gases, 6MWT, echocardiography, natriuretic peptide determination, and cardiac catheterization. Treatment usually involves oxygen therapy in addition to treatment of the underlying disease.60 A vascular phenotype has been described in COPD, characterized by hypoxemia with normocapnia or hypocapnia, very low diffusing capacity and dyspnea on minimal exertion, and a cardiovascular exercise limitation pattern in the presence of mild or moderate airflow limitation.61,62
Respiratory failureIn patients with COPD and severe hypoxemia (PaO2 ≤ 55 mmHg) at rest, continuous oxygen therapy has shown a benefit in survival and QoL. Continuous oxygen therapy (at least 15 h a day, including sleep hours) is indicated when resting PaO2 is ≤55 mmHg, and also when resting PaO2 is 56–59 mmHg with evidence of organ damage due to hypoxia (including right-sided heart failure, pulmonary hypertension and polycythemia). Oxygen flow should be sufficient to maintain a PaO2 >60 mmHg or SpO2 >90%.63
Chronic hypercapniaLong-term high-intensity home mechanical ventilation is recommended in patients with stable hypercapnic COPD, given its survival benefits, or in patients who remain hypercapnic 2–4 weeks after an episode of hypercapnic respiratory failure who require in-hospital ventilatory support due to its benefits in prolonging time to hospital readmission or death.64,65 Overlap syndrome and obesity hypoventilation syndrome should be differentiated from chronic respiratory failure attributable only to advanced COPD.
Chronic bronchitisChronic bronchitis is classically defined as the production of sputum for at least 3 months of the year in 2 consecutive years, although for practical purposes, it can be considered as the regular production of sputum in stable COPD. It is a risk factor for frequent exacerbations and has a significant impact on patient QoL.66
Patients with exacerbations and chronic bronchitis despite optimal inhaled treatment may benefit from treatment with mucolytics/antioxidants. Carbocysteine67 and high doses of N-acetylcysteine (NAC), considered antioxidant (600 mg/12 h), have shown a significant reduction in exacerbations, especially in high-risk patients (those with FEV1 <50% or 2 or more exacerbations in the previous year, or both)68–70 (Table 4).
A specific alternative for exacerbators with chronic bronchitis is roflumilast. This is an oral anti-inflammatory drug, a phosphodiesterase-4 inhibitor, that has been shown to prevent exacerbations in patients with severe COPD who have chronic cough and sputum production, and frequent exacerbations.71 This effect is maintained when roflumilast is added to maintenance treatment with an LABD, either LABA or LAMA. The effect of roflumilast in the prevention of exacerbations has been observed even when added to triple therapy, and especially in patients with more severe COPD who require hospital admission72 (Table 4). The usual dose is 500 µg orally once daily.
Adverse effects with roflumilast usually appear at the start of treatment, are rapidly detected by the patient, and usually resolve within the first four weeks, although they may occasionally lead to discontinuation of the drug. The most common adverse effects are weight loss, gastrointestinal disturbances, nausea, headache and loss of appetite. The safety profile of roflumilast is not modified by any concomitant treatment that the patient may be taking for COPD. The use of roflumilast with theophyllines should be avoided. Ges-EPOC suggests treatment with roflumilast in patients with COPD and severe obstruction, chronic bronchitis and exacerbations despite adequate inhaled treatment.70
BronchiectasisThe exacerbator phenotype can present bronchiectasis in up to 70% of cases,73 which can help perpetuate a vicious circle, amplifying the underlying inflammation and inducing the presence of frequent exacerbations, and is even associated with higher mortality.74 In patients with COPD and bronchiectasis, the infectious component and mucus hypersecretion should be treated according to bronchiectasis treatment guidelines.75
Chronic bronchial infectionChronic bronchial infection (CBI) is defined as the isolation of the same potentially pathogenic microorganism in at least 3 sputum cultures in 1 year, at least 1 month apart.76 The presence of CBI is associated with more frequent and more severe exacerbations and worse prognosis.77 Treatment will be indicated if it is associated with frequent exacerbations. There is no evidence of the effectiveness of treatment for CBI if it occurs in the rare case of non-exacerbator patients.
Long-term treatment with macrolides is indicated in exacerbator patients if they present at least 3 exacerbations in the previous year despite adequate inhaled therapy.78 Macrolides administered on a long-term basis have been shown to significantly reduce the number of exacerbations.78,79 The effectiveness of macrolides in COPD has been observed in patients with and without associated bronchiectasis.68 The recommended dose is azithromycin 500 mg/day, 3 days per week. This treatment should be reserved for reference centers with clinical, auditory, ECG, liver biochemistry and microbiological monitoring to rule out mycobacterial infection. There is little evidence on the efficacy of this treatment beyond 1 year of follow-up, so the possible risk-benefit should be evaluated annually.80 Like other international guidelines, GesEPOC suggests long-term macrolide treatment in patients with moderate to very severe COPD and exacerbations despite adequate inhaled therapy70 (Table 4).
There is very little evidence on the efficacy and safety of treatment with inhaled antibiotics, but it can be considered as an alternative in patients with severe COPD with frequent exacerbations and CBI, based on experience in the treatment of CBI in bronchiectasis.76,81
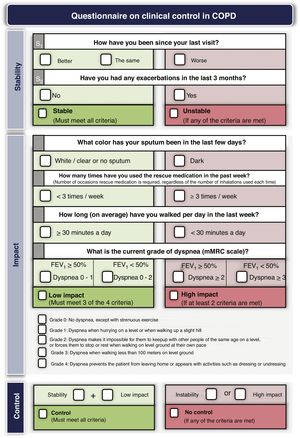
Treatment adjustment during follow-upEstablish the degree of controlTreatment should be adjusted at each medical visit, when possible changes in the risk level, phenotype or the appearance of new treatable traits should be evaluated. GesEPOC proposes to use COPD control as a therapeutic target by applying a scale designed and validated to facilitate therapeutic decisions, which is based on a series of variables easily obtained at each visit.82 The combination of these variables indicates that the patient has good control when he is in a situation of stability (no exacerbations in the previous 3 months) and low impact, defined by a low level of dyspnea, with no expectoration or mucus expectoration, infrequent use of rescue medication and an adequate level of physical activity.83 Detailed criteria for evaluating COPD control are presented in Fig. 3. Patients classified as uncontrolled have a higher risk of exacerbations both in the short term in the next 6 months, and in the long term, and a higher risk of deterioration in their health-related QoL84,85; detailed analysis of the possible causes of this lack of control is therefore necessary, and an increase in the intensity of treatment may be required.
Reduction of treatmentThe previous section on pharmacological treatment described successive steps for increasing the intensity of treatment according to the increase in symptoms or the frequency of exacerbations; conversely, de-escalation may also be considered in treatment in controlled patients. Bronchodilator treatment is known to exert its effect only during administration, so it is very likely that withdrawing a bronchodilator or replacing it with another with lower bronchodilator potency or shorter duration of action will cause functional or symptomatic worsening.86,87 Instead, GesEPOC suggests withdrawing ICS in patients who do not have frequent exacerbations (no more than 1 moderate exacerbation in the previous year) and <300 eosinophils/mm3. However, in patients with frequent exacerbations, there is insufficient evidence to establish a recommendation for withdrawal of ICS. Studies on withdrawal of ICS have shown a significant increase in the risk of exacerbations when ICS are withdrawn in patients with >300 eosinophils/mm3, so it is strongly recommended not to withdraw ICS in eosinophilic exacerbator patients88 (Table 4). The aim of ICS withdrawal is to avoid the possible appearance of adverse effects89 in patients in whom their efficacy is not proven.
Covid-19 and COPDDuring the first few months of 2020, a pandemic emerged caused by the SARS-CoV-2 coronavirus, the virus responsible for Covid-19 disease (coronavirus disease 2019).90 At the end of December 2020, more than 81 million cases had been confirmed worldwide and more than 1.9 million in Spain.
Covid-19 is a highly contagious disease with very variable clinical expression. It may be asymptomatic or present as a mild influenza-like syndrome in some people, or it can cause severe bilateral pneumonia requiring mechanical ventilation, with high mortality in severe cases.90 The sequelae are also very varied and can last for months, with manifestations in any organ or system, such as anosmia, fatigue, heart conditions, impaired concentration and sleep, or pulmonary fibrosis, among others.
Initial case series suggested that patients with COPD were underrepresented among patients with severe Covid-19, implying that they had some protective factor, perhaps derived from the COPD itself or its treatment.91 However, more recent series have reported a higher frequency of COPD cases among Covid-19 patients, although they do not appear to be any more susceptible than the general population of the same age; nevertheless, COPD may be associated with a worse prognosis.92
The SARS-CoV-2 pandemic has prompted a series of changes that profoundly affect the routine management of patients with COPD.93 A summary of the most important factors is presented in Table 6.94 In terms of the treatment of COPD, there is no evidence that any COPD treatment is either a risk factor or protects against SARS-CoV-2 infection, so both inhaled and oral treatments should remain the same as before the pandemic. Patients with COPD should comply with recommended vaccinations and protective measures, including mask use, hand hygiene and social distancing.95
Aspects to be considered in the diagnosis and treatment of COPD during the SARS-CoV-2 pandemic.
| Risk prevention | Annual influenza vaccination, mask use, hand hygiene and social distancing |
|---|---|
| Spirometry | Ask about symptoms and previous temperature measurement. Prior negative PCR if possible. Restrict to cases necessary for diagnosis or when a change in treatment may be required.98 |
| Covid-19 or COPD exacerbation | Covid-19 usually presents with fever, headache, anosmia, dysgeusia, asthenia and gastrointestinal symptoms. |
| Treatment of COPD | Maintain the same COPD treatment |
| Treatment of Covid-19 | Follow same treatment guidelines as in non-COPD patients |
| Pulmonary rehabilitation | Promote telerehabilitation programs, encourage physical activity. |
| COPD patient follow-up | Whenever possible, opt for telephone consultation, online visits or video calls. |
These guidelines have been developed and drafted with no external funding.
Conflicts of interestMarc Miravitlles has received speaker honoraria from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, AstraZeneca, Menarini, Rovi, Bial, Sandoz, Zambon, CSL Behring, Grifols and Novartis; consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Ferrer, GlaxoSmithKline, Bial, Gebro Pharma, Kamada, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, Teva, Spin Therapeutics, pH Pharma, Novartis, Sanofi and Grifols; and research grants from GlaxoSmithKline and Grifols. Myriam Calle has received honoraria for lecturing from Novartis, Chiesi, AstraZeneca, Boehringer Ingelheim and GlaxoSmithKline. Jesús Molina has received honoraria in the last 3 years for scientific advice and/or for lecturing from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini, Novartis and Pfizer. Pere Almagro has received honoraria for scientific advice and/or for lecturing from Chiesi, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Laboratorios Esteve, Menarini and Novartis. José-Tomás Gómez has received honoraria for scientific advice and/or for lecturing from AstraZeneca, Bial, Chiesi, Laboratorios Esteve, Grifols, GlaxoSmithKline, Mylan, Reig-Jofré, Rovi, Teva and Zambon. Juan Antonio Trigueros has received honoraria for training activities and participation in clinical trials from AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, GlaxoSmithKline, Mundipharma, Menarini, Pfizer and Teva. Borja G. Cosio has received honoraria for scientific advice and/or for lecturing from Chiesi, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Laboratorios Esteve, Faes Farma, Teva, Menarini, Sanofi and Novartis. Juan Antonio Riesco has received honoraria for scientific advice and/or for lecturing from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini, Mundipharma, Novartis, Pfizer, Rovi and Teva. Ciro Casanova has received honoraria in the last 3 years for lecturing and/or scientific advice and/or grants for research projects from AstraZeneca, Bial, Boehringer-Ingelheim, Chiesi, GlaxoSmithKline, Menarini and Novartis. Pere Simonet has received honoraria for continuing education activities from Boehringer Ingelheim, Menarini, Mundipharma, GlaxoSmithKline, Chiesi and AstraZeneca. David Rigau has no conflicts of interest. Jose Luis Lopez-Campos has received honoraria in the last 3 years for giving lectures, scientific advice, participation in clinical studies or writing publications for (alphabetical order): AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, Esteve, Ferrer, Gebro, GlaxoSmithKline, Grifols, Menarini, Novartis, Rovi and Teva. Joan B. Soriano has received funding for medical research and grants from 2016 to 2021 from Chiesi, GSK, Linde and Novartis via the Hospital Universitario de La Princesa Research Institute; he has participated in training activities, conferences, advisory boards and/or consultancy during the period 2015–2019 sponsored by: Air Liquide, Almirall, AstraZeneca, Boehringer-Ingelheim, CHEST, Chiesi, ERS, IHME, GEBRO, Grifols, GSK, Laminar, Linde, Lipopharma, Menarini, Mundipharma, Novartis, Pfizer, RiRL, Rovi, SEPAR, Takeda and Zambon; he has not received (directly or indirectly) funds from the tobacco industry or its affiliates. Julio Ancochea has received honoraria for scientific advice and/or for lecturing from Actelion, Air Liquide, Almirall, AstraZeneca, Boehringer Ingelheim, Carburos Médica, Chiesi, Faes Farma, Ferrer, GlaxoSmithKline, InterMune, Linde Healthcare, Menarini, MSD, Mundipharma, Novartis, Pfizer, Roche, Rovi, Sandoz, Takeda and Teva. Juan José Soler-Cataluña has received honoraria for scientific consultancy and/or for lecturing from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Grupo Ferrer, GlaxoSmithKline, Laboratorios Esteve, Teva, Menarini, Mundipharma, Novartis, Rovi and Zambon.
Coordinator: Marc Miravitlles, Spanish Society of Pulmonology and Thoracic Surgery (SEPAR). Executive Committee: Pere Almagro, Spanish Society of Internal Medicine (SEMI); Julio Ancochea, Myriam Calle, Ciro Casanova, Eusebi Chiner, Borja G. Cosío, Elena Gimeno-Santos, Carme Hernández, José Luis López-Campos, Juan Antonio Riesco, Nuria Seijas, Joan B. Soriano, Juan José Soler-Cataluña (SEPAR); Jesús Molina, Spanish Society of Family and Community Medicine (semFYC); Dolors Navarro, COPD and Sleep Apnea Patient and Family Association (APEAS), National Federation of Respiratory Patient Associations (FENAER), Spanish Patient Forum (FEP); Leopoldo Palacios Gómez, Federation of Community Nursing and Primary Care Associations (FAECAP); Pascual Piñera Salmerón, Spanish Society of Emergency Medicine (SEMES); Eulogio Pleguezuelos, Spanish Society of Rehabilitation and Physical Medicine (SERMEF), Spanish Society of Cardio-Respiratory Rehabilitation (SORECAR); Sebastià Santaeugenia, Spanish Society of Geriatrics and Gerontology (SEGG); Pere Simonet, Primary Care Respiratory Group (GRAP); Adolfo Simón, Spanish Society of Emergency Medicine (SEMES); José Tomás Gómez, Spanish Society of Primary Care Physicians (SEMERGEN); Juan Antonio Trigueros, Spanish Society of General and Family Physicians (SEMG).Methodology: David Rigau; Centro Cochrane Iberoamericano, Barcelona, Spain.
Please cite this article as: Miravitlles M, Calle M, Molina J, Almagro P, Gómez JT, Trigueros JA et al., Actualización 2021 de la Guía Española de la EPOC (GesEPOC). Tratamiento farmacológico de la EPOC estable. Arch Bronconeumol. 2022;58:69–81.
Members of the GesEPOC 2021 Working Group are listed in the Appendix A.