We report the case of a 57-year-old man with cough, dyspnea, hemoptysis, and epistaxis, and no history of exposure to drugs, toxic substances or chemicals. He had previously been diagnosed with IgG4-related disease (IgG4-RD) by lacrimal gland biopsy, and also had chronic gastritis, autoimmune hypothyroidism, hypergonadotropic hypogonadism, chronic anemia, and chronic kidney disease stage V. On physical examination, he was afebrile, tachypneic, and bleeding from the nose. Pulmonary auscultation revealed bilateral wet crackles.
Labs: RBC 3.12/mm3, Hct: 29.5%, Hb: 9.1g/dL, WBC: 15,360/mm3 (82% neutrophils), Platelets: 277,000, Glucose 109mg/dL, Creatinine: 12.46mg/dL, Urea: 161mg/dL, CPK: 72U/L, Na: 142mEquiv./L, K: 5.6mEquiv./L, PRO-BNP: 22,644pg/mL, CRP: 20.91mg/dL, PCT: 0.929ng/dL. Arterial blood gases (FiO2: 0.21): pH 7.47, pCO2: 34.4mmHg, pO2: 59mmHg, HCO3− 25.4. Normal liver function and coagulation. Chest X-ray with bilateral alveolar pattern. Chest, cranial, and upper airway CT confirmed alveolar consolidation, with thickening of left maxillary sinus mucosa (Fig. 1a).
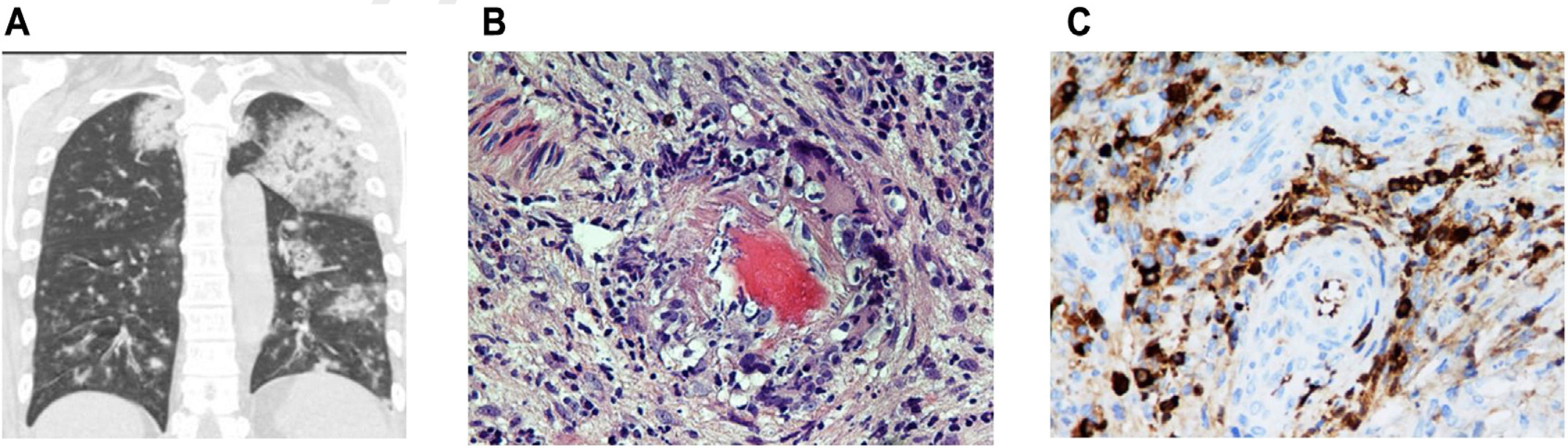
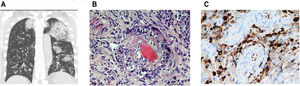
(a) Chest CT with coronal slices showing bilateral opacities associated with alveolar hemorrhage. Modified. (b) Nasal biopsy with vascular wall involvement showing vasculitis. (c) Specific immunohistochemical staining positive for IgG4 (25 IgG4-positive plasma cells per high power field) together with lymphoplasmocytic infiltrate and vascular congestion (c). Presence of vascular congestion.
Fiberoptic bronchoscopy revealed active bleeding in the left bronchial tree and bronchoalveolar lavage showed more than 15% hemosiderophages. DLCO: 120% KCO: 154%, elevated.
Autoimmune testing positive for p-ANCA (Anti-MPO: 33IU/mL) and negative for ANAs, ENAs, C3: 83mg/dL, C4: 30mg/dL, AMA, AML, Anti-MBG and cryoglobulins. Serum IgG: 934mg/dL and IgG4: 38mg/dL, normal.
Nasal biopsy (inferior and middle turbinate) showed 2 medium-caliber vascular structures with walls occupied by polymorphonuclear neutrophils, showing fibrinoid degeneration, consistent with a diagnosis of leukocytoclastic vasculitis (Fig. 1b). Specific immunohistochemical staining was positive for IgG4 (25 IgG4-positive plasma cells per high power field) together with lymphoplasmocytic infiltrate and vascular congestion (Fig. 1c). The patient was diagnosed with microscopic polyangitis-type vasculitis with p-ANCA, alveolar hemorrhage and acute renal failure, and IgG4-RD.
Treatment consisted of cyclophosphamide, prednisone (70mg/kg/day), plasmapheresis (alternating days) and hemodialysis. The patient's progress was satisfactory, with no recurrences of alveolar hemorrhage and negativization of p-ANCA. Renal function deterioration required continued hemodialysis. Four years after diagnosis, he received a renal transplant and has shown favorable progress at 1 year.
DiscussionANCA-associated vasculitis (AAV)-IgG4-RD overlap syndrome is a new entity that was first identified in 2017,1 although these findings were described in 2011 in patients with tubulointerstitial nephritis with IgG4-RD and vasculitis.2
The study by Danlos F-X et al.1 included 18 patients with a mean age of 55 years, with AAV and IgG4-RD according to Chapel Hill criteria (2013)3 and the CDC international criteria for IgG4-RD (possible, probable, and definitive).2 The diagnosis was concomitant in 72% of the series; in 17%, AAV preceded IgG4-RD and in 11%, IgG4-RD preceded AAV. The diagnosis of IgG4-RD was established as definitive in 28%, probable in 28%, and possible in 44%.1,2
Plasma replacement plus glucocorticoids was questioned in the PEXIVAS study,4 because of its lack of effectiveness in reducing mortality and preserving renal function, but the ACR 2021 consensus statement on the management of AAV recommends its use if there is a high risk of severe renal failure. Rituximab may be used as a first-line treatment in both entities.5 Multicenter studies are needed to improve knowledge and understanding of this new entity.