Pulmonary Langerhans cell histiocytosis (PLCH) belongs to the spectrum of diffuse cystic lung diseases.1 Lung involvement may be part of multisystem (MS) LCH, and may be variably associated to osteolytic bone lesions, pituitary stalk (diabetes insipidus), skin, peripheral lymph nodes LCH localisations.1 More frequently, particularly in a pulmonology setting, PLCH presents as a single LCH localisation. It is a rare sporadic disorder that occurs predominantly in young or middle-aged smokers of both genders.2
PLCH granulomas infiltrate and destroy the wall of distal bronchioles.1 Early active lesions are composed by CD1a+/CD207+ (langerin) Langerhans cells-like cells admixed with lymphocytes, macrophages, eosinophils and more rarely giant cells.1 Because PLCH may spontaneously resolve (particularly after smoking cessation) and the accumulation of immune/inflammatory cells in specific lesions, it has been long considered as a reactive granulomatous process.1
The identification in 2010 by Rollins group of the presence of recurrent somatic BRAFV600E mutations in approximately half of LCH lesions originating from different tissues, including the lung, has definitely demonstrated its clonal neoplastic nature.3 The BRAFV600E mutation induces a constitutive activation of the mitogen-activated protein kinase (MAPK) signalling pathway. This mutation is observed in various cancers, most frequently in melanomas,4 but is not synonymous of malignancy, as it may also be harboured by benign lesions.5 In their original report, Rollins and co-authors have also highlighted the constant activation of the MAPK pathway in LCH lesions, regardless of the BRAF status,3 which stimulated intense research for identifying other molecular alterations.
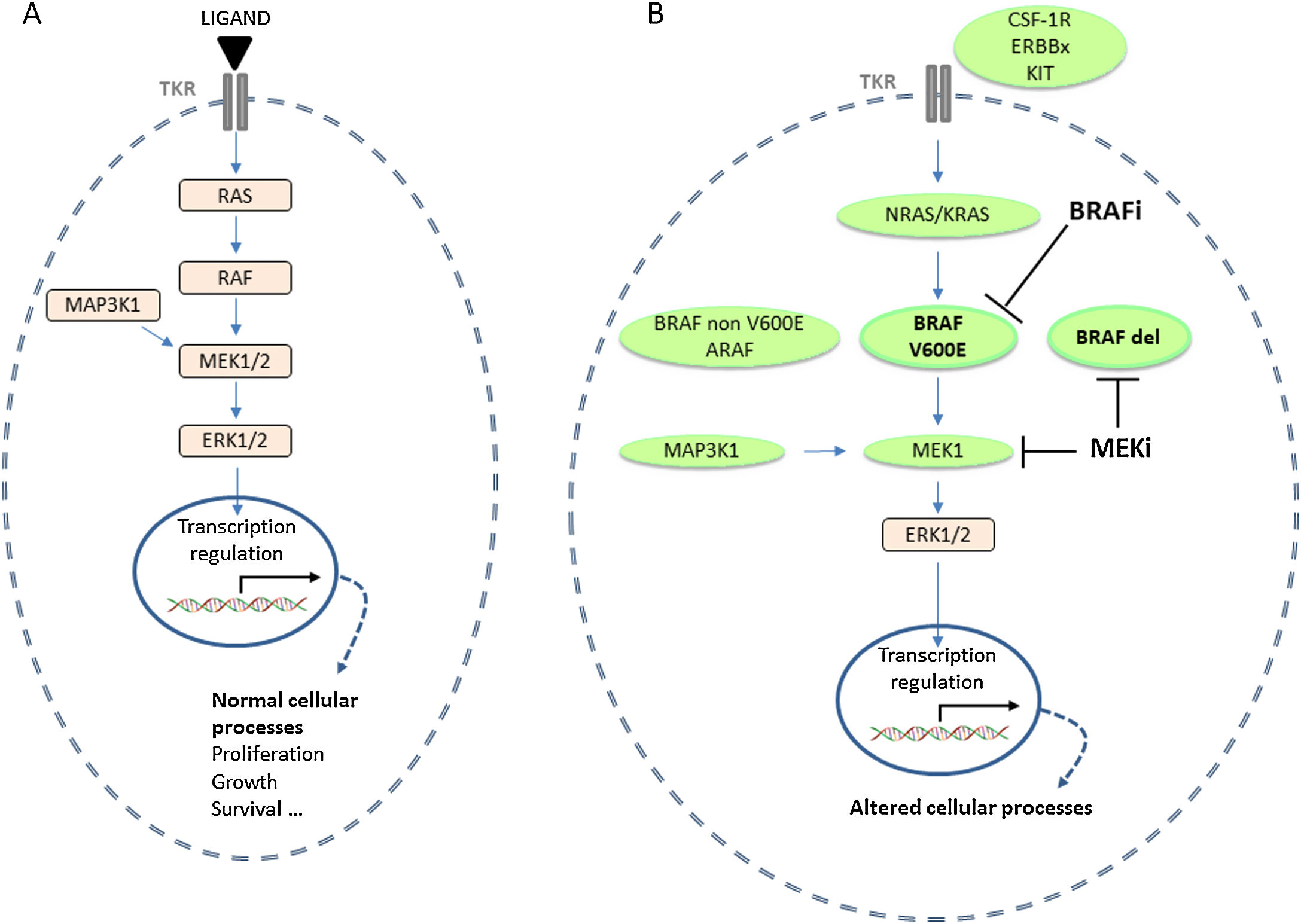
The canonical MAPK pathway is involved in several physiological cellular processes, including proliferation, differentiation, transformation, migration and survival.6 Physiologically, the MAPK pathway is activated following the interaction of a ligand with its tyrosine kinase receptor at the membrane of the cell (Fig. 1). Apart the BRAFV600E mutation, several other molecular alterations at different steps of the RAS-RAF-MEK cascade were identified in LCH lesions (Fig. 1). Our group and others have shown that the BRAFV600E mutation was present in 20–50% of PLCH lesions.7 Whereas this mutation is highly prevalent in paediatric MS LCH with involvement of the so-called risk organs (i.e. liver, spleen and bone marrow) and has an impact on the prognosis of the disease, this does not appear to be the case in adult LCH.7,8 In our series including 50 PLCH patients, we did not find any association between BRAF status and the presentation or outcome of PLCH.9
Alterations of the MAPK signalling pathway in PLCH. (A) Physiological MAPK activation. (B) Alterations of the MAPK signalling pathway and targeted therapies, BRAF inhibitors (BRAFi) and MEK inhibitors (MEKi), according to the molecular alteration. BRAFdel: BRAF deletion; ERK: extracellular signal-regulated kinase; PLCH: pulmonary langerhans cell histiocytosis; MAPK: mitogen-activated protein kinase; TKR: tyrosine kinase receptor.
The other driver mutations identified in LCH mainly affect the MEK or RAF genes. Alterations in MAP2K1 (encoding MEK1), including mutations and deletions, are observed in 9–40% of BRAF wild-type LCH lesions, and 16–20% of BRAF wild type PLCH lesions.7 MAP2K1 alterations are mutually exclusive with the presence of the BRAFV600E mutation in the lesions.7 Most alterations in MAP2K1, affect the negative autoregulatory domain in the P loop and the N-terminal catalytic core of the kinase domain of the MEK1 protein and induce constitutive activating ERK phosphorylation.7
The BRAF gene may also be affected by deletions, at the β3-αC loop in the N-terminal lobe of the kinase domain.7 This type of alterations has been first identified in a very small number of paediatric LCH, and appears more frequent in adult LCH, particularly with lung involvement.7,8,10 In our series, the BRAFN486_P490 deletion was present in 28% of PLCH lesions.9
Several other alterations have been identified in individual or small number of LCH cases and may concern ARAF, CSF-1R, ERBBx, MAP3K1, MAP3K8, KIT and BRAF (elsewhere than on codon 600) genes by point mutations but also by fusions and splicing events (Fig. 1).7
RAS mutations are rarely identified in LCH lesions.7 A particular feature of PLCH is the occurrence of NRAS mutations (Q61K/R) at low variant allele frequency in a substantial proportion of lesions, concurrently with the BRAFV600E mutation.11 The two types of mutations are carried by different clones within the lesion.11 Taken together, somatic driver mutations in the MAPK pathway are present in greater than 85% of PLCH lesions.9
Several groups worldwide have performed a tremendous work to determine the origin, development and differentiation of precursors of LCH cells that derive from bone marrow progenitors.7 Animal models have also shown that MAPK pathway is involved in myeloid differentiation, resistance to apoptosis, dysfunctional migration, and possibly senescence, which are key processes in the pathogenesis of LCH.7,12
The role of smoking in adult PLCH is an essential issue. Previous studies have shown that smoking induces the accumulation of dendritic cells and monocytes in the lungs of healthy smokers in response to various mediators, including GM-CSF, CCL20, TNF-α and TGF-β and osteopontin, which are produced in PLCH lesions.1 Liu and co-authors had shed additional light on the role of smoking in PLCH in the context of MAPK pathway activation.13 The exposure of BRAFV600E-mutated mice to cigarette smoke induced lung lesions similar to PLCH lesions, which did not occur in mice exposed to room air.13 In this BRAFV600E-mutated mice model, the accumulation of mutated cells in the lungs resulted from circulating myeloid cells in response to various chemokines.13 The LCH-like cells in lung lesions had increased viability and displayed ERK activation that could be reversed by BRAF inhibitor treatment.13 Thus, PLCH appears as a myeloid neoplastic disorder with an inflammatory component, in which smoking most likely plays a triggering role for the development of lung lesions.
The identification of molecular alterations in the MAPK pathway has generated excitement about the potential use of targeted therapies, i.e. BRAF or MEK inhibitors, according the type of mutation (Fig. 1). The efficacy of these treatments has been well demonstrated in case reports or small series of patients with severe MS LCH unresponsive to conventional chemotherapy, both in children and adults.7
MAPK pathway alteration is durably sensitive to targeted therapies, without acquired resistance in LCH.7 However, the effect of these treatments is only suspensive, requiring their prolonged maintenance. In most cases, the disease recurs after treatment cessation, although it remains sensitive when resuming the drug.7
In BRAFV600E mutated LCH lesions, BRAF inhibitors (BRAFi) of first generation (vemurafenib and dabrafenib) have shown good efficacy.7 These treatments should not be used on BRAFV600 wild type lesions, since they induce paradoxical activation of the MAPK pathway.14
MAP2K1 mutations are sensitive to MEK inhibitors (MEKi, cobimetinib and trametinib).7 BRAF deletions are less sensitive to first generation BRAFi, whereas they respond to MEKi.7 MEKi are also efficient in the absence of BRAFV600E mutation (with other mutations in MAPK pathway or no mutation identified).7 Thus, in rare situations when an urgent treatment is needed before knowledge of the mutational status of the lesion, a MEKi is the suitable targeted treatment.
The use of targeted therapy in LCH is relatively safe as in melanoma.7 Cutaneous side effects are the main concerns for BRAFi, including drug hypersensitivity syndrome or drug reaction with eosinophilia and systemic symptoms (DRESS) and squamous cell carcinomas.7 Among the potential adverse effects of MEKi, significant rhabdomyolysis, retinal detachment or impairment of cardiac function often require reduction of the treatment dose or its interruption.7 Thus, the use of these drugs in LCH remains in the field of research in expert centres.
Because adult PLCH bears an indolent course and progresses to severe lung impairment in only a minority of patients,2 the place of these targeted treatments warrants further evaluation. To the best of our knowledge, we have reported the only case of a patient with refractory PLCH whose lesion harboured a MAP2K1 deletion, and who responded durably to the MEKi trametinib.15 An international cooperative effort is highly needed in order to evaluate the effects of these treatments in a series of adult patients with PLCH.