Tobacco contributes to more than 8 million deaths each year worldwide. Quitting smoking reduces significantly the risk of death, particularly before the age of 40.1 The combination of psychological counseling with pharmacological treatment is key for a successful smoking cessation process; for years, the first-line drugs in Western Europe were nicotine replacement therapy (NRT), varenicline and bupropion,2 but currently, also cytisine should be considered as advisable pharmacological smoking cessation aid.
Here, we present the first study ever done in Spain to evaluate the role of cytisine (Todacitan®) for smoking cessation which, at the recommended doses of 1.5–9mg per day for 25 days, has been shown to be more effective than placebo and NRT in several clinical trials,3–6 and to be non-inferior and present less adverse events (AE) than varenicline.7
The present study was an observational, multicenter, multidisciplinary, cross-sectional study conducted in 7 Spanish centers, including primary care centers and hospitals. It was based on the review of real-life data from medical records and a patient's interview at the routine visit after the end of cytisine treatment (post-treatment visit). The information was collected using an electronic case report form, from March to July 2022.
The main objective was to evaluate, in routine clinical practice, the satisfaction of patients who had received cytisine for smoking cessation in the last 3 months before study initiation. The study also evaluated the tolerability and safety, the adherence and the effectiveness of cytisine for smoking cessation.
A total of 105 patients were included in the study. This sample size was lower than the initially estimated in the protocol (140), but enough to achieve the pre-defined aimed precision for the primary endpoint (±0.2 units with a SD of 1 unit).
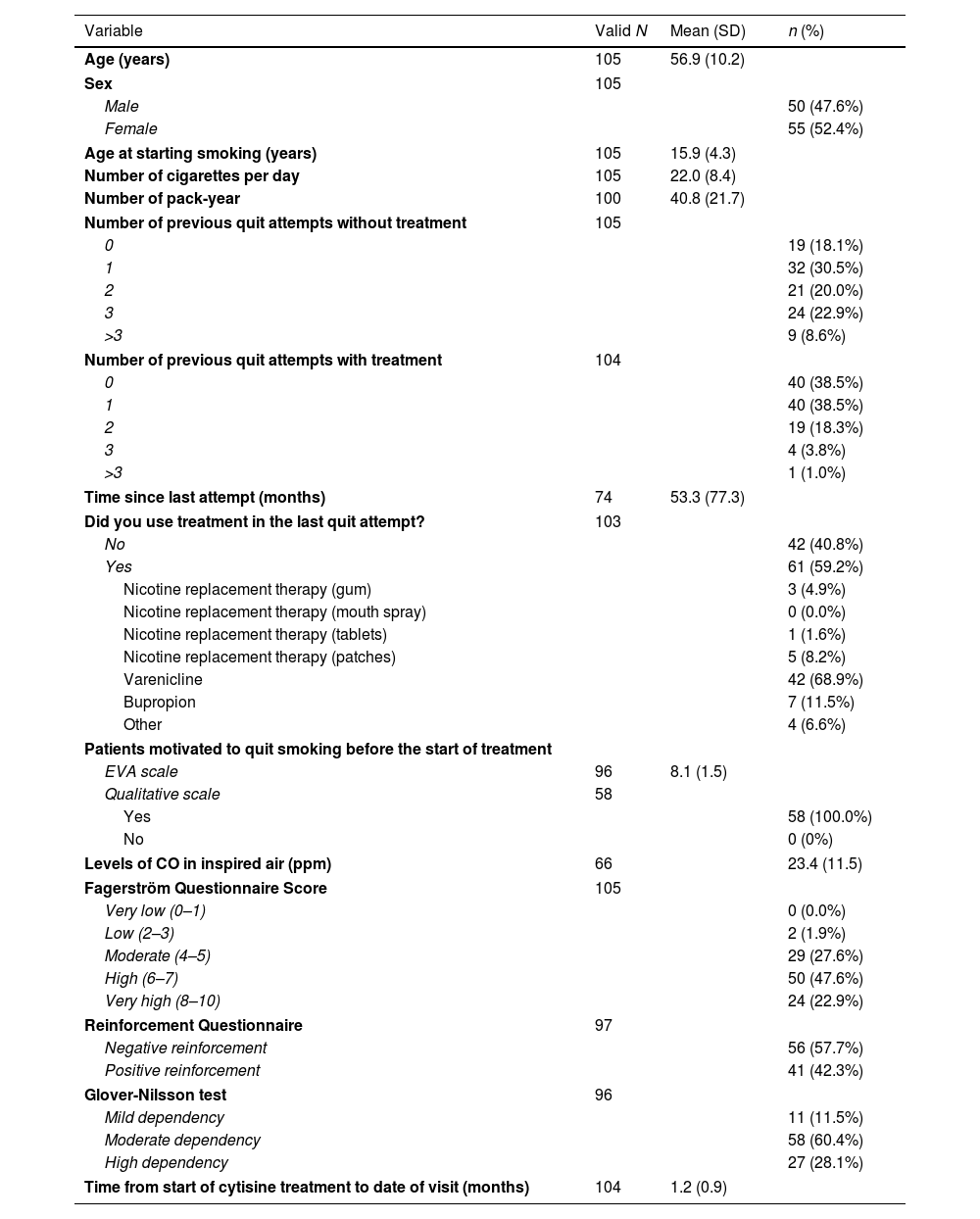
The mean age of patients was 56.9 (SD: 10.2) years; 52.4% were women. Patients were, in average, 15.9 years (SD: 4.3) old when they started smoking and, at the initial visit, they were smoking 22 (SD: 8.4) cigarettes per day and 40.8 (SD: 21.7) pack-year. A 81.9% of patients had previously tried to quit smoking; the last attempt was with pharmacological treatment in 59.2% of patients. Among the treatments used, varenicline was the most common (68.9%). 70.5% of patients had a high or very high degree of dependence to tobacco (Table 1).
Study patient's characteristics.
| Variable | Valid N | Mean (SD) | n (%) |
|---|---|---|---|
| Age (years) | 105 | 56.9 (10.2) | |
| Sex | 105 | ||
| Male | 50 (47.6%) | ||
| Female | 55 (52.4%) | ||
| Age at starting smoking (years) | 105 | 15.9 (4.3) | |
| Number of cigarettes per day | 105 | 22.0 (8.4) | |
| Number of pack-year | 100 | 40.8 (21.7) | |
| Number of previous quit attempts without treatment | 105 | ||
| 0 | 19 (18.1%) | ||
| 1 | 32 (30.5%) | ||
| 2 | 21 (20.0%) | ||
| 3 | 24 (22.9%) | ||
| >3 | 9 (8.6%) | ||
| Number of previous quit attempts with treatment | 104 | ||
| 0 | 40 (38.5%) | ||
| 1 | 40 (38.5%) | ||
| 2 | 19 (18.3%) | ||
| 3 | 4 (3.8%) | ||
| >3 | 1 (1.0%) | ||
| Time since last attempt (months) | 74 | 53.3 (77.3) | |
| Did you use treatment in the last quit attempt? | 103 | ||
| No | 42 (40.8%) | ||
| Yes | 61 (59.2%) | ||
| Nicotine replacement therapy (gum) | 3 (4.9%) | ||
| Nicotine replacement therapy (mouth spray) | 0 (0.0%) | ||
| Nicotine replacement therapy (tablets) | 1 (1.6%) | ||
| Nicotine replacement therapy (patches) | 5 (8.2%) | ||
| Varenicline | 42 (68.9%) | ||
| Bupropion | 7 (11.5%) | ||
| Other | 4 (6.6%) | ||
| Patients motivated to quit smoking before the start of treatment | |||
| EVA scale | 96 | 8.1 (1.5) | |
| Qualitative scale | 58 | ||
| Yes | 58 (100.0%) | ||
| No | 0 (0%) | ||
| Levels of CO in inspired air (ppm) | 66 | 23.4 (11.5) | |
| Fagerström Questionnaire Score | 105 | ||
| Very low (0–1) | 0 (0.0%) | ||
| Low (2–3) | 2 (1.9%) | ||
| Moderate (4–5) | 29 (27.6%) | ||
| High (6–7) | 50 (47.6%) | ||
| Very high (8–10) | 24 (22.9%) | ||
| Reinforcement Questionnaire | 97 | ||
| Negative reinforcement | 56 (57.7%) | ||
| Positive reinforcement | 41 (42.3%) | ||
| Glover-Nilsson test | 96 | ||
| Mild dependency | 11 (11.5%) | ||
| Moderate dependency | 58 (60.4%) | ||
| High dependency | 27 (28.1%) | ||
| Time from start of cytisine treatment to date of visit (months) | 104 | 1.2 (0.9) | |
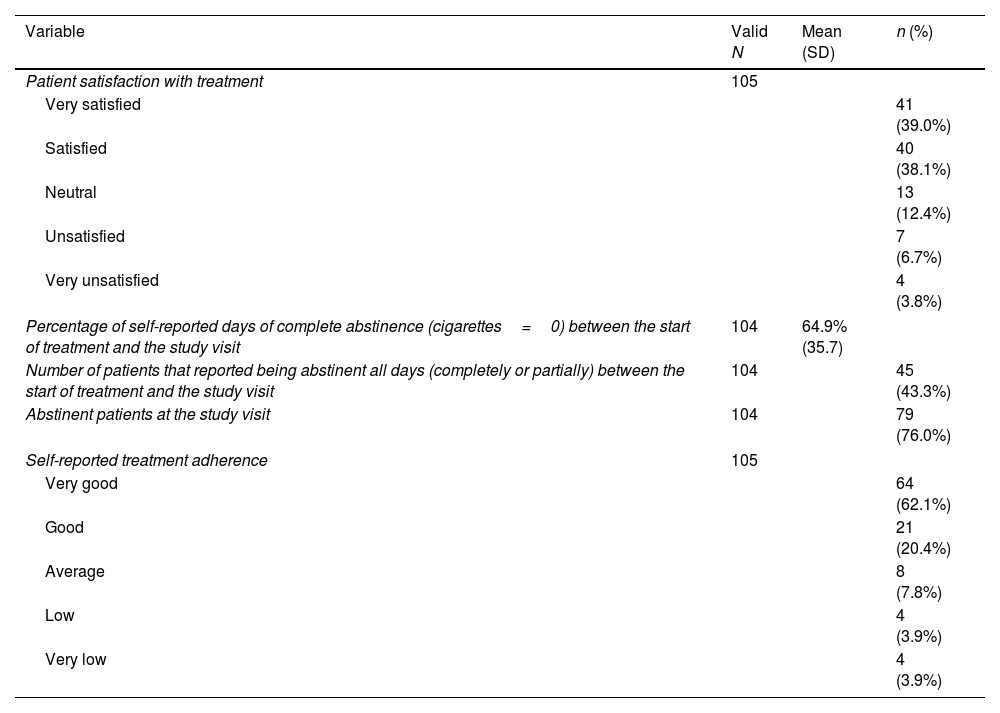
Patients were satisfied (38.1%) or very satisfied (39.0%) with the treatment. These results were independent of sex, previous treatment attempts, degree of dependence and reinforcement pattern. At the post-treatment visit, 76% of patients were abstinent. Using a Likert scale, adherence to treatment was reported as very good (62.1%) or good (20.4%). On average, patients consumed a total of 90.3 (SD: 21.1) pills over 23.2 (SD: 5.8) days of treatment (Table 2).
Results of the study for the total population.
| Variable | Valid N | Mean (SD) | n (%) |
|---|---|---|---|
| Patient satisfaction with treatment | 105 | ||
| Very satisfied | 41 (39.0%) | ||
| Satisfied | 40 (38.1%) | ||
| Neutral | 13 (12.4%) | ||
| Unsatisfied | 7 (6.7%) | ||
| Very unsatisfied | 4 (3.8%) | ||
| Percentage of self-reported days of complete abstinence (cigarettes=0) between the start of treatment and the study visit | 104 | 64.9% (35.7) | |
| Number of patients that reported being abstinent all days (completely or partially) between the start of treatment and the study visit | 104 | 45 (43.3%) | |
| Abstinent patients at the study visit | 104 | 79 (76.0%) | |
| Self-reported treatment adherence | 105 | ||
| Very good | 64 (62.1%) | ||
| Good | 21 (20.4%) | ||
| Average | 8 (7.8%) | ||
| Low | 4 (3.9%) | ||
| Very low | 4 (3.9%) | ||
The study shows that cytisine is a treatment with a manageable AE profile: 37.1% of patients experienced an AE of any degree. Sleeping disorders, usually related to smoking cessation itself, were the most common AE. Other AE included nauseas (12.4%) and vomits (3.8%). Most AE were mild (36.4%) or moderate (54.5%).
Cytisine has been shown to be more effective than placebo and NRT in several clinical trials, and to be non-inferior to varenicline and associated with fewer adverse effects.3,4,6–9 At one month, different continuous abstinence rates have been reported, including 40%6 and 59.3%9 in two independent studies conducted in New Zealand, and 57.2% in a study conducted in Italy.10 Similarly, the adverse events reported in the previous studies also include gastrointestinal events and sleeping disorders as the most prevalent ones.3,8,9
Some study limitations include the lack of concurrent control group, missing values for some variables and limited time between treatment initiation and post-treatment visit. Future studies are necessary to test the effectiveness of long-term treatment with cytisine.
In summary, the study shows that most patients (77.1%) are satisfied with the treatment with cytisine for smoking cessation and are abstinent (76%) at the post-treatment visit.
FundingThe DESTINA study and the medical writing of the scientific letter was funded by Aflofarm.
Conflict of InterestsCAJR reports grants and personal fees from Aflofarm, Gebro, GSK, Johnson&Johnson, Pfizer and Menarini. JARM reports grants and personal fees from Aflofarm, GSK, Pfizer, Novartis AG, Menarini, Boehringer Ingelheim, Astra-Zeneca, Gebro, and Laboratorios Rovi. JSCM has received honoraria as medical advisor, for presentations and publications from Aflofarm, AstraZeneca, Boerhinger, Ferrer, GSK, Menarini, Novartis, Pfizer and Rovi. JST declares to have received honoraria from Aflofarm as a clinical advisor. AMP, RSM and JLDM declare no conflicts of interest.
We thank the Adelphi Team (Maite Artés and Raquel Sales) for the management of the project and medical writing contributions.